|
|
Original Article
| ||||||
| Live animals for preclinical medical student surgical training | ||||||
| Stephanie C DeMasi1, Eriko Katsuta1,2, Kazuake Takabe1,2,3, | ||||||
|
1BS, Division of Surgical Oncology, Department of Surgery, Virginia Commonwealth University School of Medicine and Massey Cancer Center, Richmond, VA, USA.
2MD, PhD, Breast Surgery, Department of Surgical Oncology, Roswell Park Cancer Institute, Buffalo, NY, USA. 3MD, PhD, Department of Surgery, University at Buffalo, The State University of New York School of Medicine and Biomedical Sciences, Buffalo, NY, USA. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| DeMasi SC, Katsuta E, Takabe K. Live animals for preclinical medical student surgical training. Edorium J Surg 2016;5:24–31. |
|
Abstract
|
|
Aims:
The use of live animals for surgical training is a well-known, deliberated topic. However, medical students who use live animals rate the experience high not only in improving their surgical techniques, but also positively influencing their confidence levels in the operating room later in their careers. Therefore, we hypothesized that the use of live animal models is a unique and influential component of preclinical medical education.
Materials and Methods: Medical student performed the following surgical procedures using mice; surgical orthotopic implantation of cancer cells into fat pad and subsequently a radical mastectomy. The improvement of skill was then analyzed. Results: All cancer cell inoculations were performed successfully. Improvement of surgical skills during the radical mastectomy procedure was documented in all parameters. All wounds healed without breakdown or dehiscence. The appropriate interval between interrupted sutures was ascertained after fifth wound closure. The speed of interrupted sutures was doubled by last wound closure. The time required to complete a radical mastectomy decreased by almost half. A single animal died immediately following the operation due to inappropriate anesthesia, which was attributed to the lack of understanding of the overall operative management. Conclusion: Surgical training using live animals for preclinical medical students provides a unique learning experience, not only in improving surgical skills but also and arguably most importantly, to introduce the student to the complexities of the perioperative environment in a way that most closely resembles the stress and responsibility that the operating room demands. | |
|
Keywords:
Animal models, Education, Mice, Mastectomy, Surgical skills, Training
| |
|
Introduction
| ||||||
|
Medical students entering clinical rotations following two years of didactic lecturing are enthusiastic to integrate the knowledge they have gained, finally, in the clinical setting. However, many feel that medical school curricula are not meeting these amplified expectations and desires to learn these surgical skills, which students feel is an imperative component of their medical education [1] [2]. Traditionally, exposure to surgery in the preclinical years of medical school has been remarkably limited. Yet research has shown that relying solely on clerkships in third and fourth year of medical school to teach procedural skill is inadequately preparing students for their future practice [3]. Recently, it has also shown to result in a negative connotation towards of the field of surgery with consequent low application rates to categorical surgical residency programs [4]. Restriction is not only limited in time but also in its location, which conventionally has been in the operating room, which presents a unique challenge [1]. The atmosphere of the operating room invites barriers that can contribute to medical student's anxiety, low confidence, and poor self-assessment early in their clinical training [1] [5]. It has been described that the ever growing pressures and difficult working conditions in complex sub-specialized surgical fields causes dissatisfaction among young residents, let alone the medical students [6]. In addition, surgical training of preclinical medical students presents a binding concern to the safety of patients being operated on by students with limited, if any, surgical training [7]. To overcome these challenges, medical schools have implemented procedural workshops in a simulated setting to provide instruction outside of the operating room. Use of live animal models to train medical students has long been a topic of debate [6] [8]. The advantages of the use of live animals are many. They are appreciated as the best means to simulate patients in terms of preparing medical students for the anxiety and extensive perioperative demands required in the operating room, as a life is at stake even if it is an animal's [1] [6]. Some believe that animal models are beneficial for training students, as it affords an opportunity for innovation and critical thinking because the animals have very different anatomy [6]. Counter arguments on the use of live animals during student training include ethical concerns, extensive cost to obtain and house the animals, and absence of faculty to provide instruction [6]. Presently, only 1 in 5 of medical school procedural workshops use live animals [6]. However, recent studies have shown that medical students who had the opportunity to use live animals in their training rated the experience exceedingly high; not only in improving their surgical techniques, but also positively influencing their attitudes and confidence levels in the operating room later in their training, and potentially increasing their interest in surgery as a career [1] [6]. A study by Daly et al. found over 90% of students reported that they felt training in an animal laboratory was an imperative part of their education and that it should be included in the future curriculum [1] [6]. In agreement, Liddell et al. found that a single session of teaching procedural skills in the early stages of medical school may improve confidence and long-term effectiveness in basic skills [3]. With the features of current medical student education and surgical training in context, the aim of our study was to examine the effects of surgical training using mice for medical students before any clinical exposure. | ||||||
|
Materials and Methods
| ||||||
|
Participant Animal Study Tumor cell implantation model Radical Mastectomy Model Evaluation | ||||||
|
Results | ||||||
|
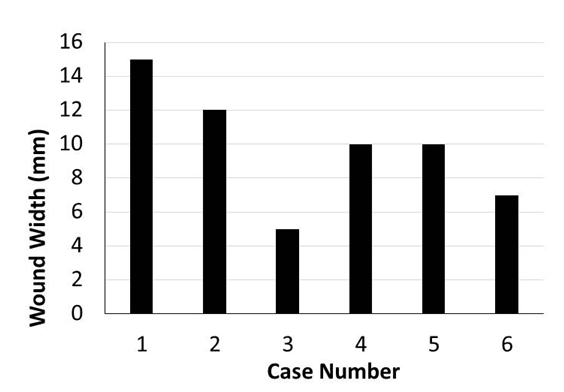
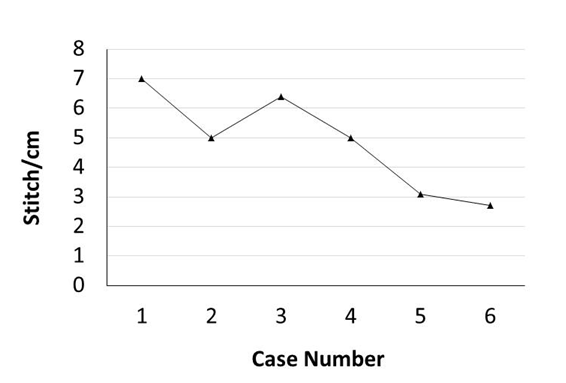
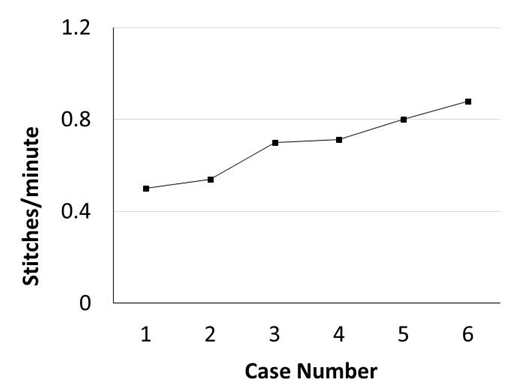
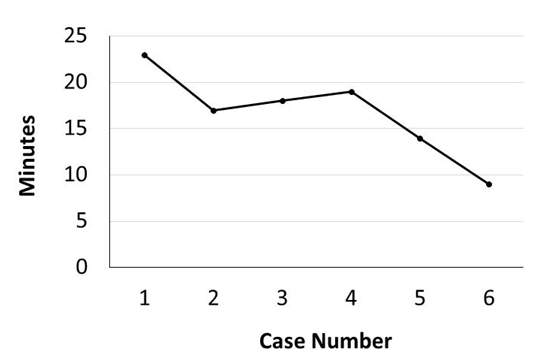
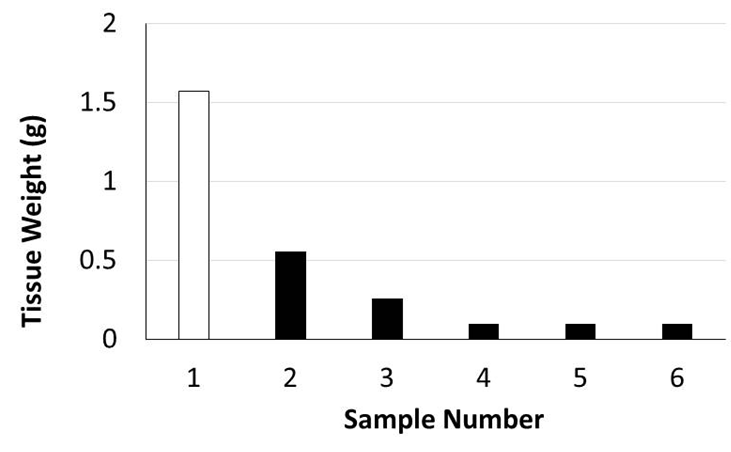
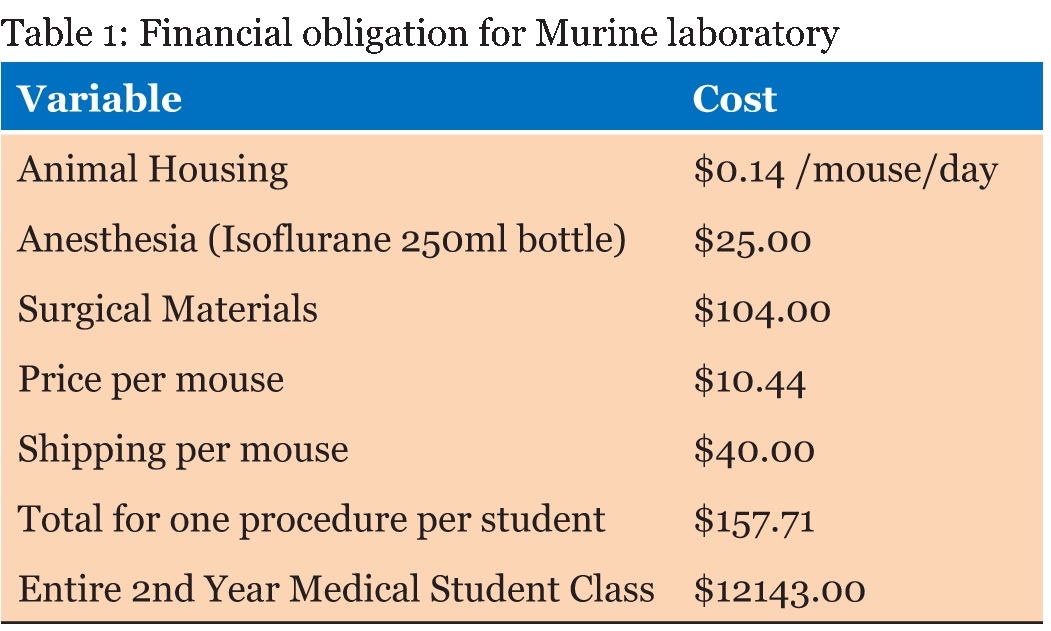
Murine orthotopic implantation under direct vision Radical Mastectomy Model The speed of suturing during the initial procedure was roughly 1 interrupted suture per 2 minutes. However, improvement was observed after each would closure, and the time to complete a successful interrupted suture decreased by half after only six wound closure experiences (Figure 4). The time to complete a radical mastectomy initially took an ample 23 minutes. As skill of suturing improved, combined with increasing comfort level and confidence with practice, improvement in time was documented with each case. It was found that the time to complete a radical mastectomy decreased by almost half by the 6th case, the final procedure took only nine minutes to complete in its entirety (Figure 5). This was a substantial decrease in time from the first operation. Perioperative Management Financial Analysis | ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
There is an undisputed requisite for basic surgical skills training in preclinical medical education. In part due to the increasing dissatisfaction of young residents coupled with the growing complexity of surgical training, as well as the imperious concern for patient safety. The lack of proper training can exacerbate the challenges presented in the high pressure environment of the operating room, which contributes to the stress, anxiety, and low self-assessment of medical students in early clinical rotations [1] [3][5] [6][11]. Medical students have steadily shown either major deficits or wide variability in basic surgical skills [6] [12][13]. The recent shortfalls of traditional training have been attributed to the financial burden, as well duty hour limitations resulting in lack of adequate time to instruct students, as teaching in the operating room can double procedure time [6] [14][15]. Medical training programs have implemented a variety of programs in an attempt to overcome these challenges. These include non-biological models, ex vivo animal tissue models, and in vivo animals in a simulated setting. Inorganic models include human simulation centers and non-biological tissue models. Some institutions offer an elective course on surgical training skills at the human simulation center to learn suturing, knot tying, laparoscopic technique, management of perioperative complications, and teamwork in the operating room. Human simulation centers have had some success in terms of objective improvement of skill, however the average start-up cost is approximately $450,000 [16] . Furthermore, annual maintenance expenditures can range from $12,000 to $300,000 [16]. A non-biological model taught flap and Z-plasty techniques using a foam rubber model [17]. However, non-biological models of teaching are encumbered with the trivial question; how closely do they truly simulate human tissue and the actual clinical environment? Though these models are cheap and easy to provide, studies have shown that non-biological models do not simulate human tissue as closely nor do they accurately represent the clinical situation when matched with animal tissue models [7] [15]. However, if compared to didactic lessons only or as a stepwise progression to animal model training it has shown that it can provide some benefit [17] [18]. Didactic lessons combined with an ex vivo pig model to teach neck dissection, radial forearm free flap, microvascular anastomosis, split-thickness skin graft, Z-plasty, and local flaps resulted improvement in surgical techniques across the board following their teaching method [7]. An ex vivo study using pig skin flaps that included stations to teach basic suturing, IV access, wound debridement, and closure of lacerations, as well as an in vivo pig model found improvements in the ex vivo objective post-assessment analysis of basic surgical skills, however concluded that in vivo models would be the ideal high-fidelity simulation environment [19]. Notably ex vivo models are a considerably cost-effective method of training, however deep skin techniques are not possible to emanate from this design [7] [19]. Large, live animal models have proven to better simulate human tissue and operating room environment as well as provide for more complex instruction and surgical technique acquisition [7]. Using live swine models that included instruction of laparotomy, small bowel resection, splenectomy, cholecystectomy, among others, found significant improvement across all post-test scores as well as positive feedback from the students whom participated [1]. There is an obligatory notion that animal models better simulate the stress and responsibility that the operating room demands. Specifically, for the past three decades it has been well recognized that poor airway management poses a serious threat to patient safety [20]. Therefore, the animal model provides a huge advantage and an additional learning curve that non-biological models, or even ex vivo models, do not offer. This was exemplified in our study as an animal's life was compromised due to that very fact and the student was able to experience the various complex components that need to be carefully managed during a surgical procedure, where a life is at stake, even if it is an animal's. This unique learning experience may in fact be more beneficial in one's training than all other objective measurements of skill acquisition combined. The limitations of large, live animal models include ethical duties, financial obligation, and absence of faculty [6]. Thus, our study aims to compromise the cost of large animal models versus non-biological models, by using a smaller murine animal model; proposing the benefits are largely equivalent. The average cost per pig alone is approximately $608, whereas mice cost roughly from $80 to $90 [1] [21]. The success of our simulated skills lab was evidenced by the significant improvement in all post-test surgical skills. The outcomes also detailed adjustment to the perioperative environment, an imperative aspect of surgical training thus proving that this model depicts a similar environment as the operating room. All surgical techniques were easily performed with practice, by the student. Though the ethical concern of the use of animal tissue still remains, one must weigh the potential compromise of patient safety when operated on by inexperienced students and/or young physicians, which also presents an ethical dilemma in it of itself. Notwithstanding, limitations are notable in this study. The first being the study was limited to a single medical student during a summer fellowship program and thus a large number of students could not participate as needed. Second, a control group comparing different surgical technique models was absent. Future investigations should include both a larger number of students to participate, as well as a control group for comparison of findings. | ||||||
|
Conclusion
| ||||||
|
The live animal model, even a small, inexpensive murine model, to train preclinical medical students provides a unique learning experience, not only to improve surgical skills, but also, and arguably most importantly, to teach the student to appreciate and understand the complexities of the perioperative environment, such as anesthesia management, that are critical to maintaining life. | ||||||
|
| ||||||
|
Acknowledgements
| ||||||
|
Kazuaki Takabe is supported by NIH/NCI grant R01CA160688 and Susan G. Komen Investigator Initiated Research Grant IIR12222224. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Stephanie C. DeMasi – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Eriko Katsuta – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Kazuaki Takabe – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2016 Stephanie C DeMasi et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|
|
About The Authors
| |||
| |||
| |||
| |||