|
|
Editorial
| ||||||
| Proposal of simple, optimal and practical operative algorithm for gastric cancer | ||||||
| Shinya Shimada1, Kenichiro Yamamoto2, Kei Horino3, Satoshi Ikeshima2, Keisuke Morita2, Hideo Baba4 | ||||||
|
1Director, Kumamoto General Hospital, Japan Community Health Care Organization, Yatsushiro, Kumamoto, Japan
2enior Surgeon, Department of Surgery, Kumamoto General Hospital, Japan Community Health Care Organization, Yatsushiro, Kumamoto, Japan 3Chief, Department of Surgery, Kumamoto General Hospital, Japan Community Health Care Organization, Yatsushiro, Kumamoto, Japan 4Professor and Chairman, Department of Gastroenterological Surgery, Faculty of Life Sciences, Kumamoto University, Japan | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Shimada S, Yamamoto K, Horino K, Ikeshima S, Morita K, Baba H. Proposal of simple, optimal and practical operative algorithm for gastric cancer. Edorium J Surg 2017;4:23–27. |
|
Despite the incidence of gastric cancer has declined in the last two decades, gastric cancer is still the third leading cause of cancer death worldwide [1][2][3] . Currently, various operative methods for patients with gastric cancer have been developed and have contributed to the individuated therapy according to the tumor stage as well as better quality of life (QoL) [4][5]. However, they have also brought calamity of complication for the choice of the appropriate treatment for gastric cancer, falling to provide inadequate operations to the patients [6][7]. Furthermore, since complete extirpation of gastric cancer with a sufficient resection margin from the tumor and removal of metastatic lymph nodes is the only treatment that offers hope of to cure the patients, practical as well as optimal treatments for gastric cancer have to be determined. By looking at the recent algorithm of standard treatments recommended by Japanese Gastric Cancer Association, the next algorithmic arm is determined by positive (cN+) or negative (cN0) lymph node metastasis followed by clinical diagnosis of T1 [8][9]. However, it is not too much to say that preoperative assessment of the status of lymph node metastases is absolutely impossible. Thus, the algorithm recommended by Japanese Gastric Cancer Association cannot be helped but said that it is out of use. On the other hand, mucosal and submucosal (SM) invasion are correctly diagnosed in 71–79.5% of the patients using endoscopic ultrasonography (EUS) [10][11], and the stratified data rather accurately showed the extent of lymph node metastasis [12][13]. Therefore, combination with accurate macroscopic diagnosis using various developed clinical examinations and microscopic diagnosis obtained by endoscopic mucosal resection and intraoperative data will lead to “practical as well as optimal treatments” for gastric cancer. From these points, it is time to propose the simple, optimal and practical therapeutic algorithm of curative operation for patients with gastric cancer, as a ubiquitous system which can be easily used by any surgeon worldwide. |
|
Clinicopathological features
| ||||||
|
The universal incidences of lymph node metastasis from tumors with mucosal, submucosal, and advanced gastric cancer are 2.3–4.9%, 17.9–23.8% and 66.0–71.2%, respectively [14] [15][16][17][18]. The detailed pathological analysis using 1051 patients with early gastric cancer treated by gastrectomy with almost all D2 dissection (extended lymphadenectomy) revealed that all mucosal tumors with lymph node metastasis (n = 14) had ulceration or ulceration scar in the lesions even when the lesion was smaller than 1.5 cm in diameter; no mucosal tumor without ulceration or ulceration scar (n = 328) had any lymph node metastasis [14]. Metastatic lymph nodes in all mucosal tumors were confined to group 1 (epigastric lymph nodes; N1). The three subgroups of submucosal had a strong positive correlation with the rate of lymph node metastasis (p < 0.001) with an incidence of 10%, 19% and 33% in SM1, SM2, and SM3, respectively. SM2 and SM3 had group 2 (distant regional lymph nodes; N2) metastasis in 3% and 7%, respectively. Further that SM1a had less lymph node involvement than that of SM1b (p = 0.04) all of which were in group 1 nodes. In advanced gastric cancer, although lymphatic and peritoneal metastasis are well known to be high, hematogenous metastasis is relatively low [19][20]. It is generally reported that approximately 60% of the metastatic tumors have distant lymph nodes (N2) metastasis [3]. Serosal invasion was also popular in advanced gastric cancer; approximately 50% of the advanced tumors had serosal invasion, falling to peritoneal dissemination. Therefore, surgical local control (tumor resection and lymph node dissection) and prophylaxis against dissemination is definitely important. | ||||||
|
Prognosis and recurrent risk
| ||||||
|
The tumor depth of gastric cancer is clearly reflected in cancer-specific five-year survival rates [3]. Gastric resection with D2 dissection for primary gastric carcinoma yielded good prognosis in mucosal and submucosal tumors; 96.0–98.8% and 91.2–94.0% of the cancer-specific five-year survival rates, respectively [14][21] [22]. There were no apparent prognostic factors in patients with mucosal tumors. In patients with submucosal tumors, the cancer-specific 5-year survival of those with lymph node metastasis was significantly lower than that of those without such metastasis. A sharp decrease in survival was seen between patients with two positive nodes and those with three positive nodes, and the cancer-specific five-year survival rate of patients with three or more metastatic lymph nodes was significantly lower than that of those with one or two nodes. Multivariate analyses revealed that the involvement of three or more lymph nodes was the sole independent prognostic determinant; the level of nodal metastasis was not an independent prognostic factor. In advanced gastric cancer, serosal invasion was the strong prognostic factor as well as the factor of the more-than-three lymph nodes metastasis. These results suggest that gastric cancer patients with more-than-three lymph nodes metastasis and serosal invasion should be given special weight of additional therapy after surgery. | ||||||
|
Prophylactic strategy (EIPL method) for peritoneal metastasis | ||||||
|
Peritoneal metastasis is the most common recurrent pattern in gastric cancer patients after curative operation. The cause of peritoneal recurrence in patients with serosa-invasive gastric cancer is the presence of intra-peritoneal free cancer cells from the serosal surface of the primary cancer and their implantation on the peritoneum. Furthermore, it has been proved that lymph node dissection opened the lymphatic channel and spread viable cancer cells into the peritoneal cavity. We have developed a powerful method for reducing the number of free cancer cells in peritoneal cavity to potentially zero, based on the law of ‘limiting dilution’, namely EIPL (Extensive Intraoperative Peritoneal Lavage). The EIPL is a very simple, little time-consuming, inexpensive and practical intra-operative technique. This therapy can easily be performed wherever and whenever, and it does not require any special techniques or devices. After the potentially curative operation, the peritoneal cavity was extensively shaken and washed, which was then followed by the complete aspiration of the fluid. This procedure was done 10 times using one liter of physiological saline. For example, ten washes of a 1:10 dilution resulted in just one cancerous cell from 1010 cells in the container based on the ‘limiting dilution theory’[23][24][25]. Thus, our prospective randomized controlled clinical trial clearly revealed that EIPL therapy significantly improved the five-year survival rate of advanced gastric cancer patients with CY1/P0 [26]. The overall five-year survival rate of the patients with EIPL group was significantly higher than that of the control groups (p < 0.0001). Among various recurrent patterns, the EIPL group had a significantly lower incidence of peritoneal recurrence than the control groups (p < 0.0001). Univariate and multivariate analyses clearly revealed that EIPL was the most significant impact factor. | ||||||
|
Proposal of the simple, optimal and practical therapeutic algorithm | ||||||
|
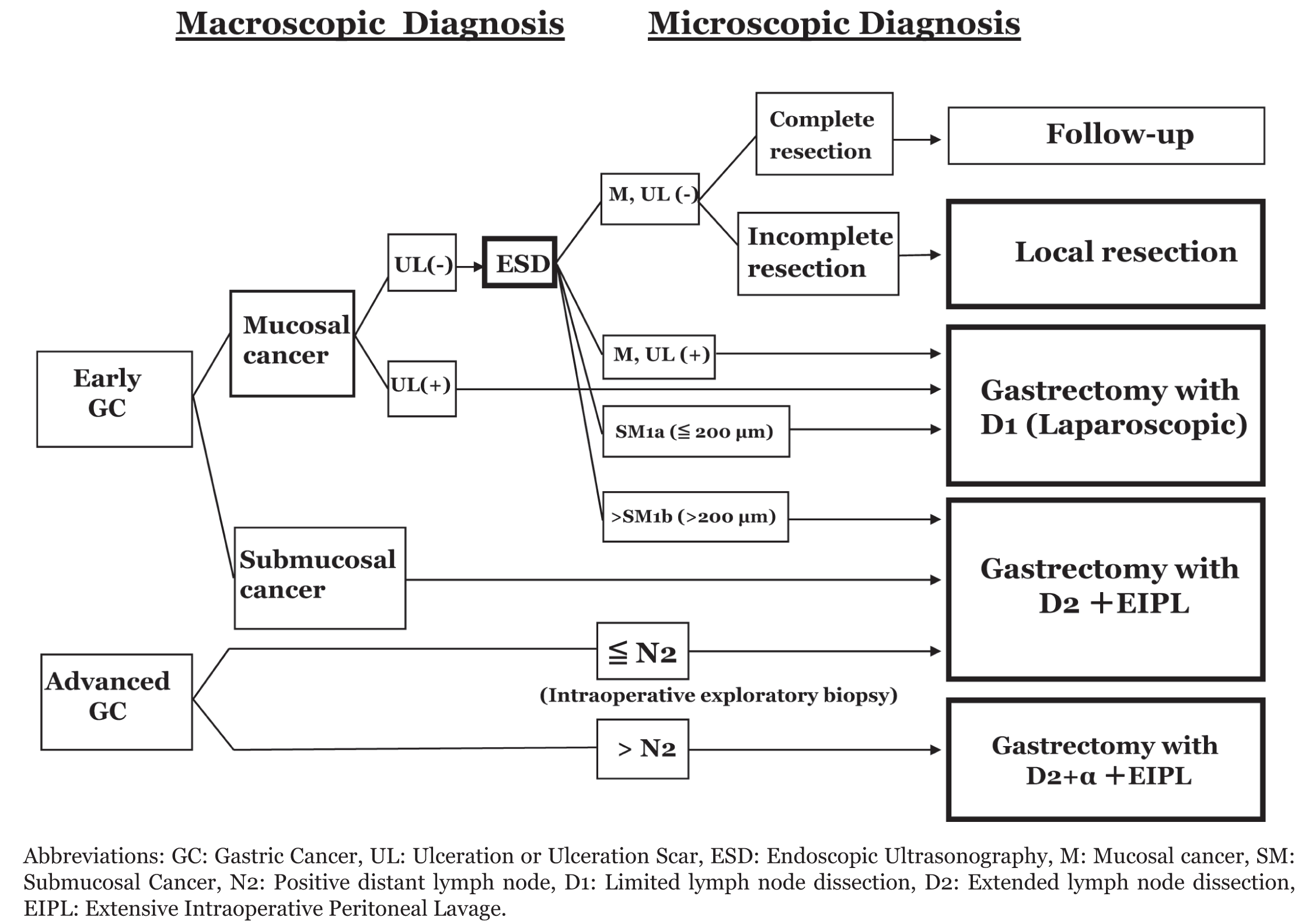
Based on the data presented in this review, the authors propose the following simple operative algorithm for gastric cancer, combined with macroscopic and microscopic diagnosis (Figure 1). Accurate diagnosis of mucosal or submucosal cancer is made macroscopically including EUS examination. All mucosal lesions without ulceration or ulceration scar should be treated by ESD. When pathologic examination of the ESD specimen reveals complete resection, the treatment is complete and the patient only needs a follow-up. If the examination reveals an incomplete resection, additional local resection is required. For mucosal tumor with ulceration or ulceration scar, or SM1a tumor, laparoscopic gastrectomy with D1 (limited lymphadenectomy) is indicated. SM1b tumors require gastrectomy with D2 dissection. Tumors macroscopically diagnosed as mucosal with ulceration or ulceration scar should be treated by laparoscopic gastrectomy with D1. All macroscopic submucosal cancer should be treated by gastrectomy with D2. Although a Dutch report has described the high postoperative morbidity and hospital mortality after gastrectomy with D2 dissection [27], D2 resections appear to be feasible and safe in Japanese [28] and selected Western patients [29]. In our study, operative morbidity and hospital mortality was 1.5% (16 of 1051) and 0.5% (5 of 1051) respectively [14]. Certain factors in the Dutch patients such as a more advanced tumor stage or larger physique comparing to those of Japanese patients in this study might have influenced the high morbidity and mortality. The present study using patients with gastric cancer suggested that the potential benefits of D2 operation outweigh the risk of increased postoperative morbidity and mortality. Therefore, advanced gastric cancer should be treated by gastrectomy with D2, and D2+a may be needed for patients with apparent N2 or N2+a metastasis from information of intraoperative exploratory biopsy. However, as a matter of course, excessive gastrectomy and lymph node dissection has to be avoided for the adverse effects on a patient’s QoL. It should be emphasized that the EIPL therapy will give better results for gastric cancer patients with serosal invasion and with lymph nodes metastasis. Intensive chemotherapy may become the key to cure gastric patients with metastatic disease and high recurrent risks such as serosal invasion and/or the more-than-three positive lymph nodes. | ||||||
| ||||||
|
CONCLUSION
| ||||||
|
In conclusion, the present review proposes the simple, optimal and practical operative algorithm for gastric cancer on the basis of the clinicopathologic and prognostic data, and our newly developed techniques. Keywords: Practical algorithm, Gastric cancer, Surgery, Treatment | ||||||
|
REFERENCES
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Shinya Shimada – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Kenichiro Yamamoto – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Kei Horino – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Satoshi Ikeshima – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Keisuke Morita – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Hideo Baba – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2017 Shinya Shimada et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|