|
Case Report
A case of accessory breast cancer mimicking metastatic lymph node from occult breast cancer
1 Senior Resident, Department of Breast Oncology and Surgery, Tokyo Medical University, Tokyo, Japan
2 Associate Professor, Tokyo Medical University Hachioji Medical Center, Tokyo, Japan
3 Associate Professor, Department of Anatomic Pathology, Tokyo Medical University, Tokyo, Japan
4 Assistant Professor, Department of Breast Surgery, Tokyo Medical University Ibaraki Medical Center, Tokyo, Japan
5 Professor, Department of Breast Oncology and Surgery, Tokyo Medical University, Tokyo, Japan
Address correspondence to:
Natsuki Uenaka
MD, Department of Breast Oncology and Surgery, Tokyo Medical University, 6-7-1 Nishishinjuku, Shinjuku-ku, Tokyo 160-0023,
Japan
Message to Corresponding Author
Article ID: 100036S05NU2019
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Uenaka N, Yamada K, Sato E, Kaise H, Ishikawa T. A case of accessory breast cancer mimicking metastatic lymph node from occult breast cancer. Edorium J Surg 2019;6:100036S05NU2019.ABSTRACT
Introduction: Primary breast cancer occurring in accessory breast tissue is rare. Presently, the diagnostic procedures and therapeutic approaches of this disease are not well established. Moreover, the rarity of this disease leads to its occasional misdiagnosis from other diseases.
Case Report: We report the case of a 69-year-old woman with accessory breast cancer who presented with a subcutaneous palpable mass in her right axillary region. The axillary mass was initially diagnosed as a metastatic lymph node from occult breast cancer at a previous hospital as her skin was intact without any sign of accessory breast. Radiological and pathological re-examination of a coreneedle biopsy (CNB) specimen from the tumor yielded a diagnosisof accessory breast cancer. We performed partial mastectomy of the mass and sentinel lymph node biopsy.
Conclusion: As this disease can be misdiagnosed and mistreated, additional clinical information regarding this disease would be highly beneficial. Although early diagnosis of accessory breast cancer may be difficult, the possibility of this disease should be considered when a palpable subcutaneous mass is detected.
Keywords: Accessory breast cancer, Lymph node, Occult breast cancer, Sentinel node biopsy
INTRODUCTION
Accessory breast tissue can occur at any site along the embryonic mammary line from the axilla to the inguinal region. This results from an incomplete embryological regression of the breast bud. The most common location of accessory breast tissue is the axilla. At six weeks of gestation, the mammary gland develops from the ectodermal ridges, also called milk line, that extend from the axilla to the inguinal region [1],[2].
Accessory breast tissue can be seen as a result of failed involution during embryogenesis except in the thoracic region [1]. Accessory breast tissue occurs in 2–6% of the general population [3],[4], with 70% occurring most commonly in the axilla [5]. Unusually, it has also appeared outside the mammary ridge, that is, in the face, posterior neck, chest, midback, buttock, vulva, flank, hip, thigh, shoulder, and upper extremities [6].
Of all breast cancers, accessory breast cancer is rare, with an incidence ranging from 0.3 to 0.6% [7]. A palpable mass is the most common physical sign of this carcinoma. Edema, tenderness, breast pain, and ambiguous discomfort are occasionally observed [1],[8]. An accessory breast may be composed of breast parenchyma, areola, and nipple, or any combination of these three components [1],[9].
We report a case of accessory breast cancer that was initially diagnosed as a metastatic lymph node from an occult breast cancer. Our findings may add further clinical information beneficial for avoiding the misdiagnosis and mistreatment of accessory breast cancer.
CASE REPORT
This case involved a 69-year-old woman with no significant past and family medical history including breast cancer. However, she noticed a painless mass in her right axillary region. Her body mass index was 30.04 (height 145.5 cm; body weight 63.3 kg). Her reason for attendance was a recently diagnosed adenocarcinoma of the breast through CNB presenting as a painless lump in the right axillary region. The mass was suggestive of a metastatic lymph node from an occult breast cancer as her breast showed no abnormal findings.
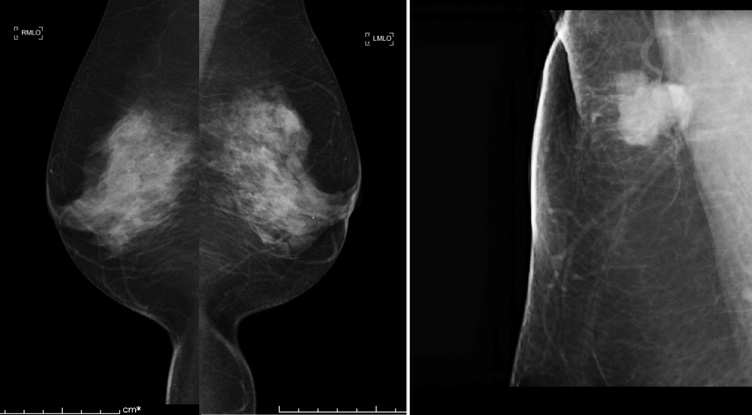
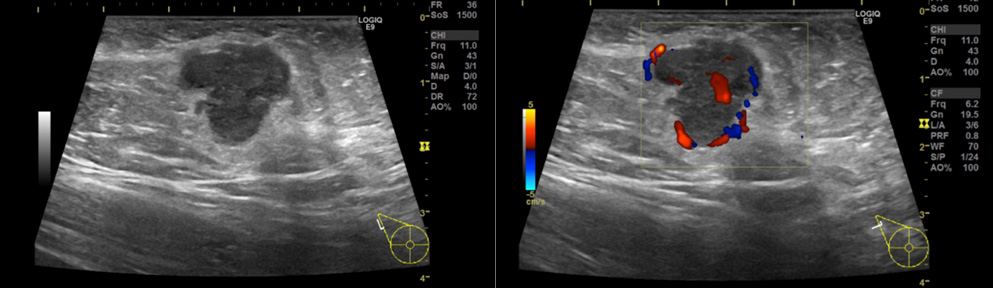
Physical examination revealed a smooth, firm, elastic movable mass measuring 20 mm in size in the right axilla. Her skin was completely intact without any sign of an accessory breast. Routine mammography (MMG) (Figure 1A) and ultrasonography (US) showed no abnormal findings in her breasts bilaterally. Additional magnified and spot-compressed axillary MMG around the palpable mass showed a high density lobular mass with a microlobulated border (Figure 1B). US detected an 18 mm microlobulated hypoechoic mass with regular margins (Figure 2A). Color Doppler US revealed mixed vascularity with both peripheral and central vessels (Figure 2B).
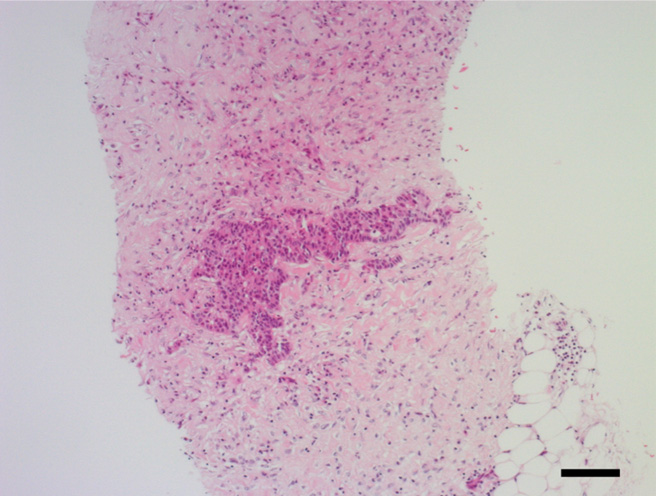
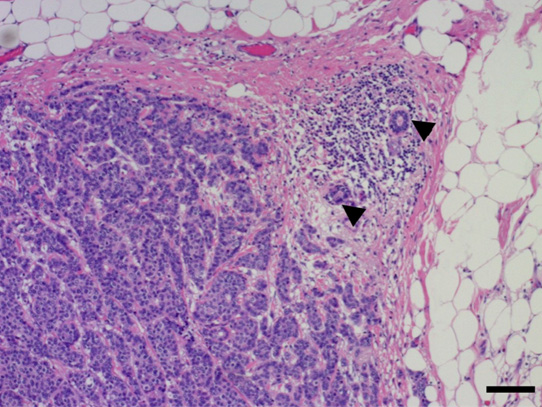
Invasive ductal carcinoma tissue was found by CNB, but non-neoplastic lactiferous ducts and lymph node structure components (e.g., lymph follicle, sinus, or capsule) were not observed (Figure 3). Because of lack of lymph node structure, suspicion of an adenocarcinoma arising from the right axillary mammary gland, that is, accessory breast cancer, arose. The TNM staging in the right axilla was cT1N0M0.
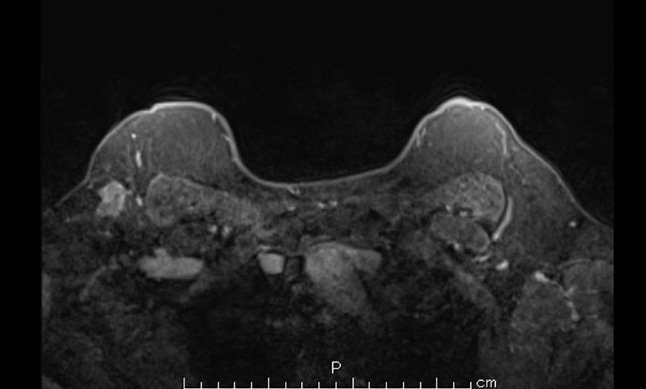
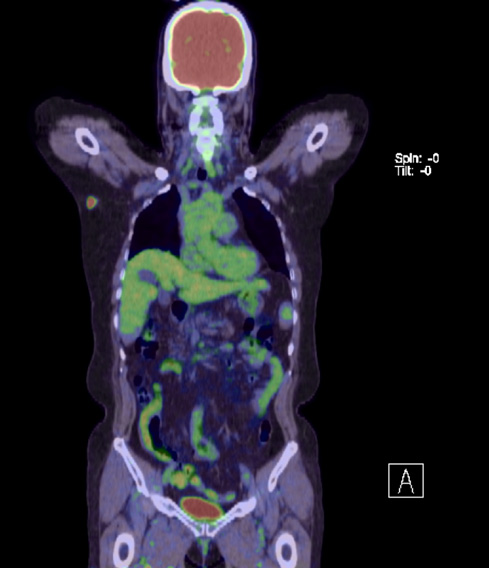
Following her diagnosis of breast cancer, magnetic resonance imaging (MRI) showed an irregular mass with a heterogeneous enhancement pattern in the right axilla. The signal intensity-time curve showed a fast washout pattern (Figure 4). Positron emission tomography-computed tomography also demonstrated a hypermetabolic mass in the right axillary region and increased FDG uptake (maximum standardized uptake value [SUVmax]= 10.24) (Figure 5). All the examinations revealed no continuity between the mass and the right mammary gland.
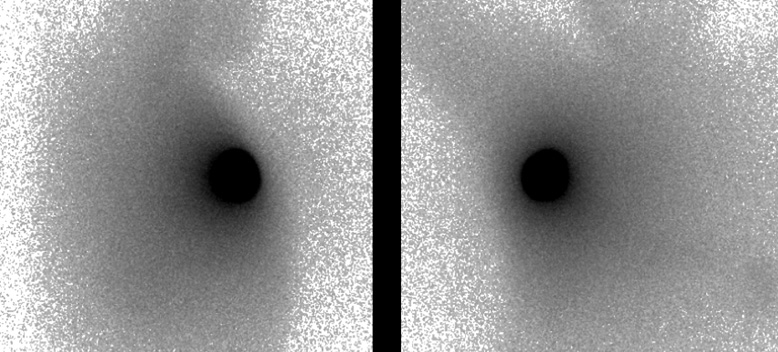
Following the diagnosis of accessory breast cancer, the patient underwent a wide resection of the right axillary mass with 1.5 cm margins and sentinel lymph node biopsy (SNB). The skin above the mass was removed by spindle-shaped resection. 99mTechnetiumradiolabeled phytate was injected into the right areola six hours before surgery. Indigo carmine was injected into the right areola 10 minutes before surgery. It was difficult to identify the axillary sentinel lymph node because of the “shine through” effect (Figure 6). The gamma probe identified no radioactive lymph node, and there were no blue dye-filled tract and node in the right axilla. However, several lymph nodes were easily detected and sampled because of a good view in the axilla by skin incision for the wide resection. The frozen section diagnosis of lymph nodes was negative, thus axillary dissection was not performed.
The definitive pathological diagnosis based on the surgical specimen was accessory breast cancer consisting of solid-tubular carcinoma of nuclear grade 3, forming a 1.6×1.2 cm tumor (Figure 7) and (Figure 8). There were no signs of lymph-vascular invasion. The surgical margins were free from cancer and the six resected lymph nodes were all negative. The TNM staging of the lesion was pT1N0M0. Immunohistochemical staining showed that the tissue was positive for both estrogen receptor and progesterone receptor, and negative for human epidermal growth factor 2. Ki-67 staining showed a proliferative index of 30%. Postoperatively, the patient was started on endocrine therapy with anastrozole, which was planned to be taken for five years. Radiotherapy was declined by the patient.
DISCUSSION
In this case, a palpable mass was found only in the right axillary region without any other skin symptoms. Thus, the mass was initially misdiagnosed as a metastatic lymph node from occult breast cancer.
When accessory breast cancer is presented without the nipple or areolar complex, a definitive diagnosis of carcinoma arising from the accessory breast tissue in or outside the axilla can be difficult to make, and it is frequently misdiagnosed as a subcutaneous lesion [10],[11]. The clinical differential diagnosis of an axillary mass may include other subcutaneous masses, such as neurofibroma, lipoma, lymphoma, hidradenitis suppurativa, and follicular cysts [1],[12]. There are also metastatic lymph nodes from breast cancer and subsequently from other malignancies, such as lung cancer, digestive tract cancer, and ovarian cancer. Notably, accessory breast cancer is also one of the most commonly reported palpable axillary masses [13],[14],[15]. Accessory breast cancer should also be carefully considered when a mass is found in the axillary region even if there are no accompanying skin findings.
Accessory breast cancer is usually treated by a wide resection of the tumor together with the surrounding tissue, covering skin, and regional lymph nodes [5],[16],[17]. Currently, there are a few studies on SNB for accessory breast cancer [18],[19],[20]. As accessory breast cancer is located in the axilla, the radioactive colloid often causes diffusion of radioactivity, or the “shine through” effect which makes SNB difficult [4],[21]. An unpredictable lymphatic drainage has also been reported in the accessory breast [4], which may subsequently hinder successful SNB mapping. We could not identify lymph nodes by dual mapping with the radioisotope and dye. However, several lymph nodes were easily identifiable for sampling.
Although endocrine therapy was expected to be added as an adjuvant therapy, irradiation of the whole breast in accordance with the routine practice for breast cancer was questionable. Thus, we considered partial irradiation of the resected area. Although the middle- and long-term benefits, safety, and indications of adjuvant radiotherapy have not yet been fully evaluated for accessory breast cancer [1], irradiation of the axilla tumor site appears to decrease local recurrence [1],[4]. In the present case, however, radiotherapy was not performed in accordance with the patient’s preference.
CONCLUSION
The early diagnosis of accessory breast cancer may prove difficult because of the rareness of this disease, particularly when it presents without the nippleareolar complex. Accessory breast cancer should be carefully taken into consideration in cases of a palpable subcutaneous mass. As identification of a sentinel node using the routine mapping method is difficult, careful sampling is required for cases of clinically negative nodes.
REFERENCES
1.
Zhang S, Yu YH, Qu W, Zhang Y, Li J. Diagnosis and treatment of accessory breast cancer in 11 patients. Oncol Lett 2015;10(3):1783–8. [CrossRef]
[Pubmed]

2.
Visconti G, Eltahir Y, Van Ginkel RJ, Bart J, Werker PM. Approach and management of primary ectopic breast carcinoma in the axilla: Where are we? A comprehensive historical literature review. J Plast Reconstr Aesthet Surg 2011;64(1):e1–11. [CrossRef]
[Pubmed]

3.
Rho JY, Juhng SK, Yoon KJ. Carcinoma originating from aberrant breast tissue of the right upper anterior chest wall: A case report. J Korean Med Sci 2001;16(4):519–21. [CrossRef]
[Pubmed]

4.
Dhebri A, Shah N, Sripadam R, Aora PK. Skin lesion in axilla: An unusual presentation of invasive lobular carcinoma of breast. BMJ Case Rep 2012;2012. pii: bcr2012006642. [CrossRef]
[Pubmed]

5.
Youn HJ, Jung SH. Accessory breast carcinoma. Breast Care (Basel) 2009;4(2):104–6. [CrossRef]
[Pubmed]

6.
Hao JY, Yang CC, Liu FF, et al. Accessory breast cancer occurring concurrently with bilateral primary invasive breast carcinomas: A report of two cases and literature review. Cancer Biol Med 2012;9(3):197–201. [CrossRef]
[Pubmed]

7.
Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: A case report. Case Rep Med 2012;2012:286210. [CrossRef]
[Pubmed]

8.
Pardo M, Silva F, Jiménez P, Karmelic M. Mammary carcinoma ine ectopic breast tissue. A case report. [Article in Spanish]. Rev Med Chil 2001;129(6):663–5.
[Pubmed]

9.
Page RN, Dittrich L, King R, Boulos F, Page DL. Syringomatous adenoma of the nipple occurring within a supernumerary breast: A case report. J Cutan Pathol 2009;36(11):1206–9. [CrossRef]
[Pubmed]

10.
Burdick AE, Thomas KA, Welsh E, Powell J, Elgart GW. Axillary polymastia. J Am Acad Dermatol 2003;49(6):1154–6. [CrossRef]
[Pubmed]

11.
Rho JY, Juhng SK, Yoon KJ. Carcinoma originating from aberrant breast tissue of the right upper anterior chest wall: A case report. J Korean Med Sci 2001;16(4):519–21. [CrossRef]
[Pubmed]

12.
Jordan K, Laumann A, Conrad S, Medenica M. Axillary mass in a 20-year-old woman. Diagnosis: Axillary accessory breast tissue. Arch Dermatol 2001;137(10):1367–72.
[Pubmed]

13.
Xu R, Li J, Zhang Y, Jing H, Zhu Y. Male occult breast cancer with axillary lymph node metastasis as the first manifestation: A case report and literature review. Medicine (Baltimore) 2017;96(51):e9312. [CrossRef]
[Pubmed]

14.
Park JE, Sohn YM, Kim EK. Sonographic findings of axillary masses: What can be imaged in this space. J Ultrasound Med 2013;32(7):1261–70. [CrossRef]
[Pubmed]

15.
Copeland EM, McBride CM. Axillary metastases from unknown primary sites. Ann Surg 1973;178(1):25–7. [CrossRef]
[Pubmed]

16.
Marshall MB, Moynihan JJ, Frost A, Evans SR. Ectopic breast cancer: Case report and literature review. Surg Oncol 1994;3(5):295–304.
[Pubmed]

17.
Livesey JR, Price BA. Metastatic accessory breast carcinoma in a thoracic subcutaneous nodule. J R Soc Med 1990;83(12):799–800.
[Pubmed]

18.
Alavifard R, Kadkhodayan S, Homaee Shandiz F, Dabbagh VR, Sadeghi R. Is sentinel node mapping possible in surgically removed ectopic axillary breast cancer? A case report. Nucl Med Rev Cent East Eur 2016;19(B):29–30. [CrossRef]
[Pubmed]

19.
Francone E, Nathan MJ, Mureli F, Bruno MS, Traverso E, Friedman D. Ectopic breast cancer: Case report and review of the literature. Aesthetic Plast Surg 2013;37(4):746–9. [CrossRef]
[Pubmed]

20.
Thorne AL, Jackson A, Yiangou C. The use of sentinel node biopsy in the treatment of cancer of an accessory breast. Breast 2003;12(2):153–5. [CrossRef]
[Pubmed]

21.
Chagpar A, Martin RC 3rd, Chao C, et al. Validation of subareolar and periareolar injection techniques for breast sentinel lymph node biopsy. Arch Surg 2004;139(6)614–8. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgments
We thank Dr. Edward Barroga (https://orcid.org/0000- 0002-8920-2607) for reviewing and editing the manuscript.
Author ContributionsNatsuki Uenaka - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Kimito Yamada - Analysis of data, Interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published
Eiichi Sato - Analysis of data, Interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published
Hiroshi Kaise - Analysis of data, Interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published
Takashi Ishikawa - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2019 Natsuki Uenaka et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.