|
Case Report
Inflammatory perforated cecal tumor due to intestinal histoplasmosis in an immunocompetent patient
1 Postgraduate Medical Doctor, PGY4 General Surgery, General Enrique Garcés Hospital, Quito, Ecuador
2 General and Laparoscopic Surgeon, General Enrique Garcés Hospital, Quito, Ecuador
3 Resident Infectology Physician, Baca Ortiz Hospital, Quito, Ecuador
4 Postgraduate Medical Doctor, PGY2 General Surgery, General Enrique Garcés Hospital, Quito, Ecuador
Address correspondence to:
Jhony A Delgado
Passage OE9B S12-26 and Los Canelos, Chilibulo, Quito,
Ecuador
Message to Corresponding Author
Article ID: 100045S05SO2020
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Ordoñez SR, Aguilar BE, Delgado JA, Arboleda GA, Medina VR, Valladares AE. Inflammatory perforated cecal tumor due to intestinal histoplasmosis in an immunocompetent patient. Edorium J Surg 2020;7:100045S05SO2020.ABSTRACT
In this case report, the case of a patient who presents with abdominal pain and acute appendicitis signs and symptoms is highlighted. This patient underwent surgery and a cecal tumor with perforations was observed intraoperatively. An oncological surgical approach was decided due to macroscopic evidence. Furthermore, a later histopathological result of intestinal histoplasmosis which is a rare pathology that frequently occurs in immunosuppressed patients was detected. However, in this rare case, the middle-aged male patient does not present immunosuppression problems.
Keywords: Cecal tumor, Immunocompetent patient, Intestinal histoplasmosis
INTRODUCTION
Histoplasmosis is an infection caused by a fungus called Histoplasma capsulatum [1]. Its dissemination to the gastrointestinal tract is infrequent as it occurs in 1 of 5000 cases of histoplasmosis. Even rarer, its presence in patients with preserved immunity reaches 0.05% [2]. Indeed, the most frequent symptoms are diarrhea, abdominal pain, and weight loss [3]. Additionally, the gastrointestinal location is pathophysiologically caused by the large amount of lymphoid tissue in this system. The clinical treatment is Amphotericin B or ketoconazole. However, despite being a specific treatment, relapses have been detected when applied. Noteworthy, surgical treatment is reserved for complications that manifest an acute abdomen due to intestinal perforation.
CASE REPORT
A 24-year-old male patient residing in Sacha (Orellana, eastern Ecuador), without significant pathological history, came to our hospital due to the presence of moderate intensity colic-type abdominal pain that had lasted 14 hours. It began in the mesogastrium and it was later located in the right iliac fossa. In addition, the patient had vomited four times without apparent cause.
Upon admission, vital signs as 130/70 mm Hg blood pressure, 95 beats per minute heart rate and 37 °C temperature were measured. In fact, an abdomen with positive appendicular signs (McBurney, Blumberg, Dunphy) drew attention in the physical examination. Therefore, laboratory examinations were carried out showing 18,200 leukocytosis with neutrophilia (85.5%) and elevated C-reactive protein (CRP) of 6.
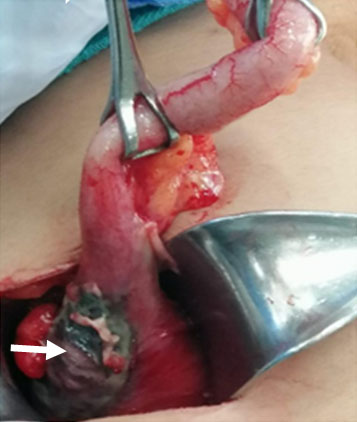
Emergent surgical resolution (appendectomy) was decided. An opened approach was performed with a McBurney-type incision. Afterward, inflammatory fluid of approximately 100 mL was observed. Additionally, the presence of a cecum mass of approximately 4 × 3 cm was detected. This mass deforms the cecum and retracts it. Indeed, partial necrosis with perforations showing evidence of leakage, gas, and purulent liquid was found (Figure 1).
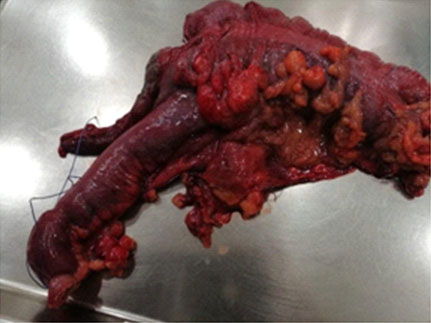
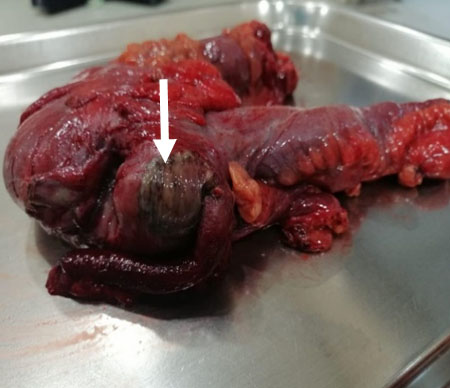
Given the suspicion of a malignant neoplasm, the applied surgery was a laparotomy where multiple pericolic, epiploic nodes, in the root of the mesentery, the largest up to 2 cm in diameter, were found with no evidence of tumor implants in the liver or peritoneum. Due to the aforementioned cecum mass, a right hemicolectomy and lymph node emptying with low vascular pedicle ligation (right colic) and latero-lateral ileo-transverse anastomosis were performed (Figure 2 and Figure 3). Then, the patient was managed with a postsurgical diagnosis of perforated cecal tumor.
Further exams such as serology tests [enzyme-linked immunosorbent assay (ELISA)], tumor markers cancer embryonic antigen (CEA), and carbohydrate antigen (CA19-9) were negative, without pathological findings in computed tomography of the chest, abdomen, and pelvis.
The patient evolved adequately, and he was discharged on the fourth postoperative day with adequate intestinal transit and good tolerance to the enteral diet.
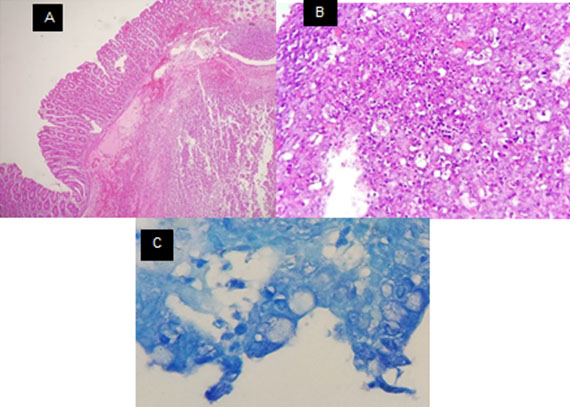
The pathology report macroscopically revealed a tumor with necrotic areas and microscopically showed intestinal mycosis compatible with histoplasmosis, negative for malignancy (Figure 4). An evaluation from the infectious disease service was requested. Furthermore, serology and viral load with western blot for suspected acquired immunodeficiency disease were performed and these analyses were negative. Treatment with Amphotericin B started at a dose of 1 mg per kg of weight per day for one week, continued later with itraconazole 200 mg each day for 12 months. Being the treatment of choice, the patient evolved clinically and surgically in an appropriate way. The patient is currently being followed up by Internal Medicine.
DISCUSSION
Histoplasmosis is a granulomatous disease caused by an intracellular dimorphic fungus called H. capsulatum, for which soil is the natural habitat [1],[4].
The infection is acquired by inhalation of the microconidia found in the feces of birds and bats. These microconidia reach the pulmonary alveoli where they are phagocytosed by alveolar macrophages and then microconidia are spread to the entire mononuclear phagocytic system. It is the most common endemic mycosis in individuals infected with human immunodeficiency virus [1],[5].
In South American countries, it is endemic to eastern areas [6]. This would be the main risk factor that can be associated with the patients. Histoplasmosis disseminated in the gastrointestinal tract occurs in 1 of 5000 cases. Histoplasmosis has been well documented in the literature within the immunocompromised population. Furthermore, the gastrointestinal condition is rare in a patient who does not have his immunological system compromised. The incidence of which represents 0.05% of the cases. In fact, symptoms are absent in the majority of cases. Therefore, diagnosis is complicated [2],[7].
The presence of disseminated histoplasmosis with gastrointestinal involvement is common in patients with CD4+ cell counts of <200 cells/mm3 [8].
As described, the main symptoms are diarrhea, weight loss, and abdominal pain [3]. Indeed, it is commonly presented as a bowel mass, ulcer, or polypoid lesion in the cecum and terminal ileum [5].
The localization at the gastrointestinal level is a consequence of the large amount of lymphoid tissue present there. Some patients’ reports mention its presence as an acute abdomen setting confused with acute appendicitis [3].
Histopathologically, the differential diagnosis of histoplasmosis should include other bacterial and fungal infections. In low magnification, Hematoxylin and Eosin (H&E) preparations, H. capsulatum and Leishmania are very similar. Consequently, Toxoplasma may resemble Histoplasma superficially. However, it is smaller, and it is usually not seen within macrophages. Any of the special fungal stains will clarify the difference between Histoplasma, Leishmania, and Toxoplasma successfully [7].
At the West China Hospital, Sichuan University, an intestinal study was carried out from October 2005 to March 2015, where four immunocompetent patients were diagnosed endoscopically. These researchers concluded that histoplasmosis presence in immunocompetent patients may exist without being reported since most fungi tests are not routinely performed [8].
The manifestations mimic many other gastrointestinal diseases, such as Crohn’s disease, tuberculosis, carcinomas, and lymphomas. Histoplasmosis is not often considered as a cause in the differential diagnosis, which leads to inappropriate therapies [8],[9].
The prognosis for disseminated histoplasmosis is poor: 84% died in a study prior to development of Amphotericin B. Although Amphotericin B kills the organism in vivo, a relapse may occur after therapy completion [8],[9],[10].
Ketoconazole is associated with fewer side effects. In 1981, Hawkins reported excellent responses in four patients with disseminated histoplasmosis. Mabey reported a successful treatment of one patient with histoplasmosis who was treated with 200 mg of ketoconazole daily. Slama found that 13 of 14 immunocompetent patients with progressive or disseminated cavitary histoplasmosis were cured with 200 mg of ketoconazole daily [8],[9].
A treatment with 200 mg of itraconazole is taken orally twice a day for three days, and then 1 or 2 times a day for three months are reasonable. However, when considering disseminated histoplasmosis, it is recommended to be applied for 12–24 months [10].
Medical management of gastrointestinal histoplasmosis is recommended and surgical resection in case of urgent cases is reserved for acute emergencies such as intestinal perforation or when required for diagnosis [8].
CONCLUSION
Histoplasmosis is a frequent infection in immunocompetent people that generally does not present symptoms. However, when symptoms appear, treatment is clinically performed administering antifungals. When a patient presents an acute abdomen with signs of intestinal perforation, his treatment is surgical and antifungal treatment is applied for a long time because the infection is widespread.
REFERENCES
1.
Kauffman CA. Histoplasmosis: A clinical and laboratory update. Clin Microbiol Rev 2007;20(1):115–32. [CrossRef]
[Pubmed]

2.
Romano R, Soape MM, Thirumala S, Ghandour E. Disseminated histoplasmosis mimicking metastatic disease of the colon and omentum: Report of a case and literature review. Arab J Gastroenterol 2015;16(2):66–8. [CrossRef]
[Pubmed]

3.
4.
5.
Alva E, Vásquez J, Frisancho O, Yaza M, Yábar A. Colonic histoplasmosis as a diagnostic manifestation of AIDS. [Article in Spanish]. Rev Gastroenterol Peru 2010;30(2):163–6.
[Pubmed]

6.
Naniwadekar A, Malhotra A. Education and imaging. Gastrointestinal: Gastrointestinal histoplasmosis. J Gastroenterol Hepatol 2008;23(4):668. [CrossRef]
[Pubmed]

7.
Sharma R, Lipi L, Gajendra S, et al. Gastrointestinal histoplasmosis: A case series. Int J Surg Pathol 2017;25(7):592–8. [CrossRef]
[Pubmed]

8.
Zhu LL, Wang J, Wang ZJ, Wang YP, Yang JL. Intestinal histoplasmosis in immunocompetent adults. World J Gastroenterol 2016;22(15):4027–33. [CrossRef]
[Pubmed]

9.
Soto Tarazona AR, Meza Flores JL, Garrido Rivadeneyra D, Cok Garcia J. Gastric histoplasmosis simulating a malignant gastric ulcer. [Article in Spanish]. Rev Gastroenterol Peru 2003;23(3):221–4.
[Pubmed]

10.
Kahi CJ, Wheat LJ, Allen SD, Sarosi GA. Gastrointestinal histoplasmosis. Am J Gastroenterol 2005;100(1):220–31. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Stalin R Ordoñe - Substantial contributions to conception and design, Interpretation of data, Drafting the article, Final approval of the version to be published
Bernabé E Aguilar - Revising it critically for important intellectual content, Final approval of the version to be published
Jhony A Delgado - Acquisition of data, Revising it critically for important intellectual content, Final approval of the version to be published
Gabriela A Arboleda - Acquisition of data, Drafting the article, Final approval of the version to be published
Victor R Medina - Interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published
Andrea E Valladares - Acquisition of data, Analysis of data, Drafting the article, Final approval of the version to be published
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2020 Stalin R Ordoñez et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.