|
Case Report
Management of submucosal posterior gastric GIST by endoscopic assisted laparoscopic wedge gastrectomy - A modification of classical laparoscopic and endoscopic cooperative surgery (LECS)
1 Department of surgical gastroenterology, Madras Medical College, Chennai, India
Address correspondence to:
J M V Amarjothi
Department of Surgical Gastroenterology, Madras Medical College, Chennai, 600003,
India
Message to Corresponding Author
Article ID: 100035S05JA2019
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Amarjothi JMV, Villalan R, Jeyasudhahar J, Babu OLN. Management of submucosal posterior gastric GIST by endoscopic assisted laparoscopic wedge gastrectomy - A modification of classical laparoscopic and endoscopic cooperative surgery. Edorium J Surg 2019;6:100035S05JA2019.ABSTRACT
Laparoscopy and endoscopy can be used in tandem for tumors in difficult locations like submucosal gastrointestinal stromal tumor (GIST) in the posterior wall of the stomach. This combined approach ensures accurate localization of the tumors resulting in negative margins which would be much difficult to obtain by conventional laparoscopic approach alone. Endoscopic localization of tumor helps not only in accurate localization but also in observing for staple line integrity. The aim of the article is to show the use of both techniques in the management of submucosal GIST in a difficult location like posterior gastric wall.
Keywords: Combined Surgery, Endoscopy, Submucosal GIST
INTRODUCTION
Submucosal gastrointestinal stromal tumors (GISTs) are difficult to localise laparoscopically. Hence a combination of approaches like endoscopy and laparoscopy in tandem help in better localization and confirm excision completion. We describe a case where such a combined approach was used.
CASE REPORT
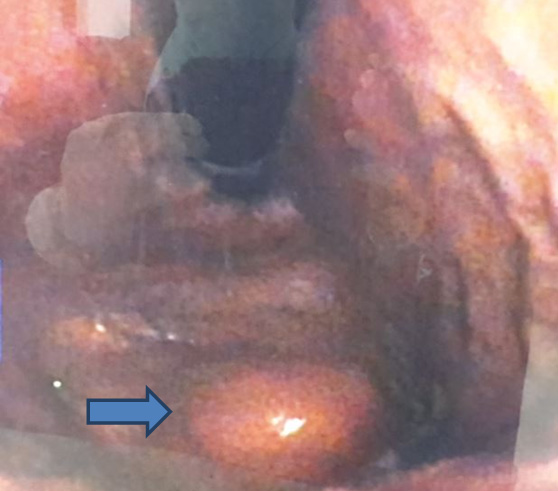
A 65-year-old female presented with intermittent abdominal pain, dull aching, non-colicky not radiating to back with increased intensity for one week. Clinical examination was normal. UGI endoscopy showed polypoidal growth in the greater curvature of stomach at junction of antrum and body. The endoscopic biopsy was non-specific (Figure 1).
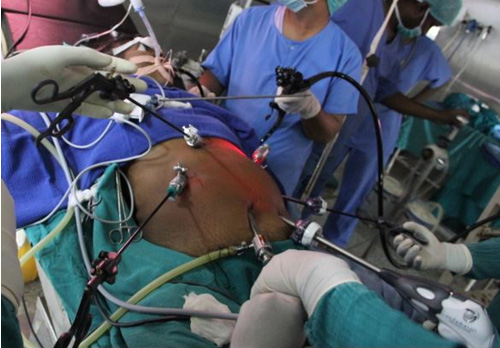
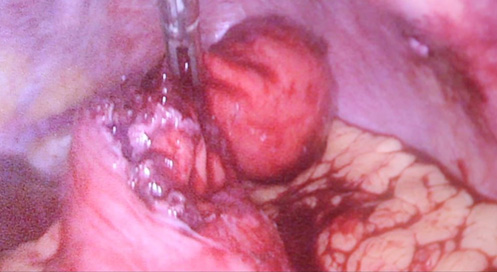
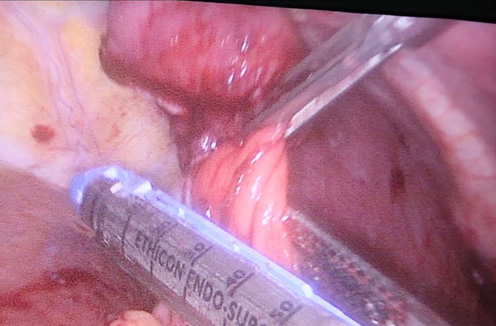
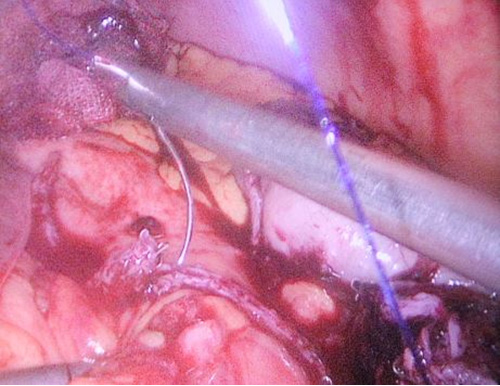
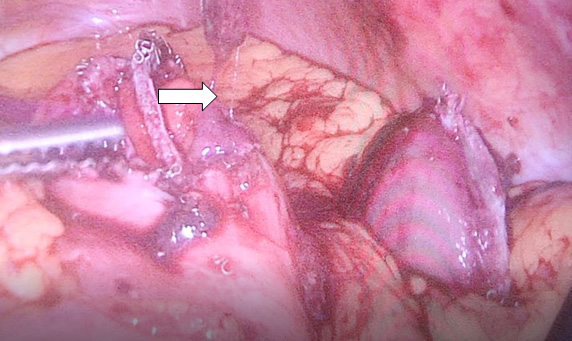
Contrast enhanced computed tomography (CECT) abdomen showed irregular nodular thickening involving the lesser and greater curvature of stomach with no evidence of significant lymphadenopathy or metastasis. It was decided to go for combined approach (laparoscopic and endoscopic) as there was a possibility of this being a gastric GIST. After creating Veress pneumoperitoneum, the following ports were made: 10 mm umbilicus for camera, 12 mm for left lumbar working+specimen extraction port, three 5 mm working ports in the epigastrium, left hypochondrial and right lumbar regions. Patient was in semi lithotomy, head up, position with a pneumoperitoneum of 10 mm Hg. The greater omentum was opened to identify the tumor. However, it was not localised laparoscopically. Intraoperative endoscopy was done (Figure 2) with the endoscopic operator positioned at the top of the patient’s head and position of the polyp was confirmed. The endoscopy enabled the tumor to be detected on the posterior wall of the stomach and also enabled planning the gastrotomy site on anterior wall. Gastrotomy was done on the anterior wall of stomach and polyp was grasped laparoscopically and traction was applied (Figure 3). Endostapler 60 mm blue load was applied at base of polyp and then wedge resection of the greater curvature margins of the polyp done (Figure 4). The staple line was observed to confirm its integrity and to exclude any deformity before being over sewn (Figure 5), (Figure 6). The gastrotomy incision site was then elevated and the incision line was closed with another laparoscopic stapler (Figure 7). Total operation time was 200 minutes and blood loss was 15 ml.
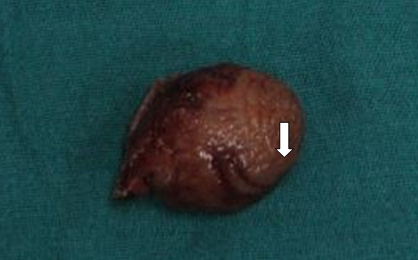
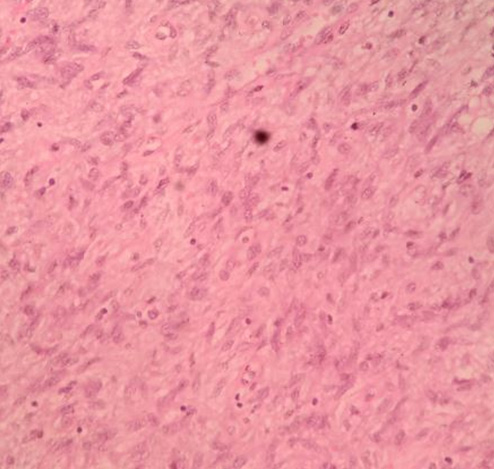
Specimen (Figure 8) containing gastric polyp and wedge margins was extracted in rubber extraction bag through the 12 mm port by extending the port site. Hemostasis was then secured. 12 mm 10 mm ports were closed in two layers with 1-vicryl and skin with 3-0 ethilon. In the other ports, skin closure was done. Histopathological examination was suggestive of low grade GIST with cells arranged in fascicular pattern with oval to spindle shaped tumor cells, moderate eosinophilic cytoplasm and oval vesicular nuclei which exhibited moderate pleomorphism (Figure 9). Mitosis was 3-4 /HPF (High Power Field). Immunohistochemical staining was positive for CD117, and negative for CD34 and desmin. As patient had features of low grade gastric GIST, patient was given no adjuvant therapy. Patient is on follow up with no recurrence.
DISCUSSION
Laparoscopic surgery is associated with low morbidity and mortality, decreased hospital stay due to quick recovery, reduced pain and better oncologic outcomes. It is also non-inferior to open surgery even in the long term [1],[2]. In a large meta-analysis of 28 trials between open and laparoscopic surgery for GIST, there were no significant differences between both thereby placing the onus on better patient selection and right intraoperative decisions [2]. Laparoscopic wedge resections for GIST, in particular are associated with better short term and long term outcomes when compared to open surgery [3].
In 2004, National Comprehensive Cancer Network (NCCN) GIST Task Force and the GIST Consensus Conference under the auspices of The European Society for Medical Oncology (ESMO) showed that laparoscopic resection may be used for small gastric GISTs (<2 cm in size) [4]. Current ESMO guidelines do not mention a size criteria but only describe that if laparoscopic excision is planned, the technique needs to follow the principles of oncological surgery [5] and that such a laparoscopic approach is clearly discouraged in patients who have large tumors, because of risk of tumor rupture, which is associated with a very high risk of relapse. However, laparoscopic GIST resection even for large lesions above 5 cm, is considered a safe and acceptable technique making size relatively redundant [6].
However, many single-institution studies have restricted laparoscopic resections to patients with otherwise favorable tumor characteristics (2–5 cm, without ulcer formation or locally invasive disease), as determined by preoperative endoscopy with or without endoscopic ultrasound and computerized tomographic (CT) imaging. These studies exclude patients with tumors at the esophagogastric junction or pylorus [7].
Gastrointestinal stromal tumors (GISTs) are most common in the stomach and arise from the interstitial cells of Cajal. The majority of GISTs (approximately 95%) express the CD117 antigen (KIT), a proto-oncogene product about 95% of these neoplasms have mutations in the c-KIT gene. Gastric GIST may be symptomatic and present with abdominal pain and bleeding especially if submucosal. Preoperative histological diagnosis of submucosal gastric lesions is however not always feasible through endoscopic biopsy [8]. In fact, the latest recommendations and NCCN guidelines do not make prior histological confirmation of GIST mandatory and are required only if neoadjuvant treatment is planned [9].
Since gastric GISTs rarely metastasize to lymph nodes, there is no need for lymphadenectomy. In order to achieve adequate oncologic resection, 1–2 cm free margin is recommended [10], [11]. So treatment of choice would be simple laproscopic wedge resection for gastric GISTs. This is aided by the development of endoscopic stapling devices and the evidence that laparoscopic resection of GISTs is associated with minimal morbidity [12]. Histopathologic pattern of GISTs is usually fusiform followed by epithelioid and mixed types [13].
Laparoscopic excision is reserved for GISTs located on the anterior wall of the stomach where though easy to perform, it may result in excessive gastric resection with resultant deformity of the stomach. Therefore, laparoscopic wedge resection is currently used for small tumors on the anterior wall of the stomach and is not universally adopted for large tumors or for those in other locations, such as the esophagogastric junction area or the pyloric zone [14].
Posterior submucosal GISTS are difficult to locate and may be associated with increased morbidity especially in the obese [15]. To facilitate accurate localization of the tumor, a preoperative endoscopic marking or preferably, an endoscopic rendezvous or laparoscopic ultrasound or even a gastrotomy especially in intramural tumours can be used [3].
To allow precise resection using intraluminal endoscopy, some methods have been described. They include exposure techniques like classical laparoscopic and endoscopic cooperative surgery (Classic LECS) and inverted LECS and non-exposure techniques like combination of Laparoscopic and Endoscopic Approaches to Neoplasia with Non-Exposure Technique [CLEAN NET] and non-exposed Endoscopic Wall-inversion Surgery (NEWS). Exposure implies exposure to gastric content and tumour cells due to gastric perforation. LECS has an inherent risk of peritoneal infection due to the necessity for gastric perforation. However, CLEANNET and NEWS have been developed to prevent the risk of cancer cells seeding during open gastrectomy. These procedures might thus have potential for minimally invasive resections of gastric tumors, even those in an ulcerated state [16].
It is to be noted that techniques like classic LECS and inverted LECS are highly dependent on expertise for therapeutic endoscopic techniques like Endoscopic Submucosal Dissection (ESD). When such expertise is unavailable, simple techniques like using endoscopy more as a visual diagnostic tool [14] as in our case can be used. Endoscopy is necessary to confirm the tumor location, to incise the gastric wall without damaging the tumor, ensure hemostasis, to verify that no other malignant transformation is evident from the mucosal side of the stomach and to exclude an anastomotic leak immediately after the procedure. When comparing LECS group to conventional laparoscopic resection group, it was associated with lower blood loss and longer operative time than the laparoscopic group [17].
CONCLUSION
Combined laproscopic and endoscopic procedures can be used in the accurate management of gastric GISTs especially in inaccessible locations like in the posterior gastric wall when compared to conventional laproscopy and achieve better margins.
REFERENCES
1.
Samardzic J. Laparoscopic wedge resection of gastric stromal tumor (GIST). Med Arch 2015;69(3):203–5. [CrossRef]
[Pubmed]

2.
Ye L, Wu X, Wu T, et al. Meta-analysis of laparoscopic vs. open resection of gastric gastrointestinal stromal tumors. PLoS One 2017;12(5):e0177193. [CrossRef]
[Pubmed]

3.
Catena F, Di Battista M, Fusaroli P, et al. Laparoscopic treatment of gastric GIST: Report of 21 cases and literature’s review. J Gastrointest Surg 2008;12(3):561–8. [CrossRef]
[Pubmed]

4.
Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol 2005;16(4):566–78. [CrossRef]
[Pubmed]

5.
ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii21–6. [CrossRef]
[Pubmed]

6.
Loureiro Mde P, Almeida RA, Claus CM, et al. Laparoscopic resection of gastrointestinal stromal tumors (GIST). [Article in Portuguese]. Arq Bras Cir Dig 2016;29(1):1–4. [CrossRef]
[Pubmed]

7.
Sexton JA, Pierce RA, Halpin VJ, et al. Laparoscopic gastric resection for gastrointestinal stromal tumors. Surg Endosc 2008;22:(12)2583–7. [CrossRef]
[Pubmed]

8.
Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol 2002;3(11):655–64.
[Pubmed]

9.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. 2008. [Available at: https://www.nccn.org/professionals/ physician_gls/default.aspx]

10.
Rosen MJ, Heniford BT. Endoluminal gastric surgery: The modern era of minimally invasive surgery. Surg Clin North Am 2005;85(5):989–1007. [CrossRef]
[Pubmed]

11.
Yano H, Kimura Y, Iwazawa T, et al. Hand-assisted laparoscopic surgery for a large gastrointestinal stromal tumor of the stomach. Gastric Cancer 2005;8(3):186–92. [CrossRef]
[Pubmed]

12.
Kitamura Y. Gastrointestinal stromal tumors: Past, present, and future. J Gastroenterol 2008;43(7):499–508. [CrossRef]
[Pubmed]

13.
Patil DT, Rubin BP. Gastrointestinal stromal tumor: Advances in diagnosis and management. Arch Pathol Lab Med 2011;135(10):1298–310. [CrossRef]
[Pubmed]

14.
Funahashi H, Miyai H, Wakasugi T, et al. Successful gastrointestinal stromal tumor resection using modified laparoscopic and endoscopic cooperative surgery: Report of a case. J Gastroint Dig Syst 2014;4:204. [CrossRef]

15.
Ronellenfitsch U, Staiger W, Kähler G, Ströbel P, Schwarzbach M, Hohenberger P. Perioperative and oncological outcome of laparoscopic resection of gastrointestinal stromal tumour (GIST) of the stomach. Diagn Ther Endosc 2009;2009:286138. [CrossRef]
[Pubmed]

16.
Mitsui T, Niimi K, Yamashita H, et al. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer 2014;17(3):594–9. [CrossRef]
[Pubmed]

17.
Namikawa T, Hanazaki K. Laparoscopic endoscopic cooperative surgery as a minimally invasive treatment for gastric submucosal tumor. World J Gastrointest Endosc 2015;7(14):1150–6. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions Guaranter of Submission
The corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2019 J M V Amarjothi et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.