|
Research Article
Impact of neoadjuvant and adjuvant chemotherapy on postoperative complications after mastectomy with immediate breast reconstruction
1 Department of General Surgery, Zagazig University, Zagazig, Egypt
Address correspondence to:
Hassan A Saad
Department of General Surgery, Zagazig University, Zagazig,
Egypt
Message to Corresponding Author
Article ID: 100040S05HS2019
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Saad HA, El Teliti AM, Arafa AS, Abdelbari A. Impact of neoadjuvant and adjuvant chemotherapy on postoperative complications after mastectomy with immediate breast reconstruction. Edorium J Surg 2019;6:100040S05HS2019.ABSTRACT
Aim: Use of chemotherapy has a big role in cancer breast treatment. This study is done to determine the impact and timing of neoadjuvant and adjuvant chemotherapy on postoperative outcomes after mastectomy with immediate breast reconstruction. This study was done in Zagazig University Hospitals, Egypt.
Methods: Retrospective study including 82 patients underwent mastectomy and immediate breast reconstruction with Intervention Systemic neoadjuvant and adjuvant chemotherapy for breast cancer during the period of study (January 2017 to December 2018), with a postoperative follow-up of two years at Zagazig University Surgical Department.
Results: Nine patients (45%) in the adjuvant chemotherapy developed postoperative infections, compared with seven patients (25%) in the neoadjuvant chemotherapy group and 8 patients (24%) who did not receive any chemotherapy (p = 0.05). Overall, 30% of patients had a complication requiring an unplanned return to the surgical room.
Conclusion: The adjuvant chemotherapy group had high rate of infected wound but there were no differences between groups with respect to unplanned reoperation, donor-site complications, or expander loss.
Keywords: Cancer breast, Chemotherapy, Neoadjuvant, Reconstruction
INTRODUCTION
Breast reconstruction (BR) is becoming a new option of care in the management of breast cancer patients at time of surgical removal of cancer to decrease the possible spread and improve cosmetic outcome. The recently completed National Mastectomy and Breast cancer Reconstruction Audit (NMBRA) involving more than 18,000 women examined a broad range of clinical and patient outcomes reported mortality and survival. The audit also looked at important factors as information and access to reconstructive technique, as well as degree of pain, complications, life quality, and well-being experienced by women following a variety of procedures had been done. Today, the radical mastectomy is rarely performed, however, with breast cancer affected women; oncoplastic surgery remains an important part of breast cancer treatment, and especially for more advanced or locally aggressive tumors [1]. Multiple studies explained the benefits of immediate reconstruction after tumor excision which improves psychological and aesthetic outcomes of patients [2]. From oncological and surgical view immediate reconstruction is safe [3], without any difference in complications when compared with delayed reconstruction [4]. We performed a prospectively collected outcomes study of women who underwent this procedure and effect of chemotherapy received to detect wound and other adverse reactions. Neoadjuvant chemotherapy plays an important role in modern breast cancer technique [5]. It reduces the size and recurrence rates in both breast and axilla, female may achieve complete resections with less extensive destructive operations [6],[7],[8], the neoadjuvant therapy has both prognostic and prescriptive true value. Treatment response is predictive of long-term survival, but no response to the therapy may inform future chemotherapy choices [9],[10]. The use of neoadjuvant chemotherapy may have an impact on the timing of reconstruction [11]. Multiple studies have examined the influence of irradiation after mastectomy and large studies have shown increased wound infection and cosmetic results after breast reconstruction in patients who receive chemotherapy [12]. The proven efficacy of neoadjuvant and adjuvant chemotherapy for improving oncologic outcomes and survival rate in breast but the effect of chemotherapy on breast cosmetic outcomes is not well proved [13],[14]. With the rise in the mastectomy rate, there has been a resultant increase in the number of patients who choose to undergo post-mastectomy reconstruction [15],[16].
MATERIALS AND METHODS
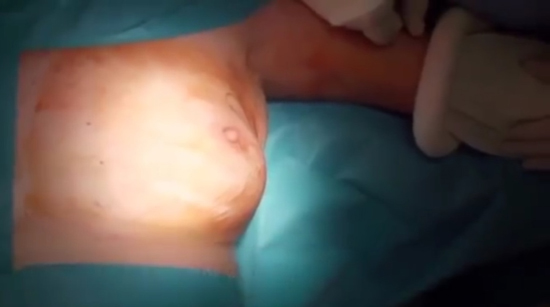
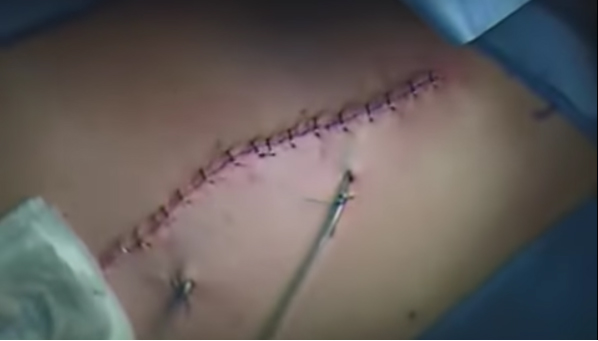
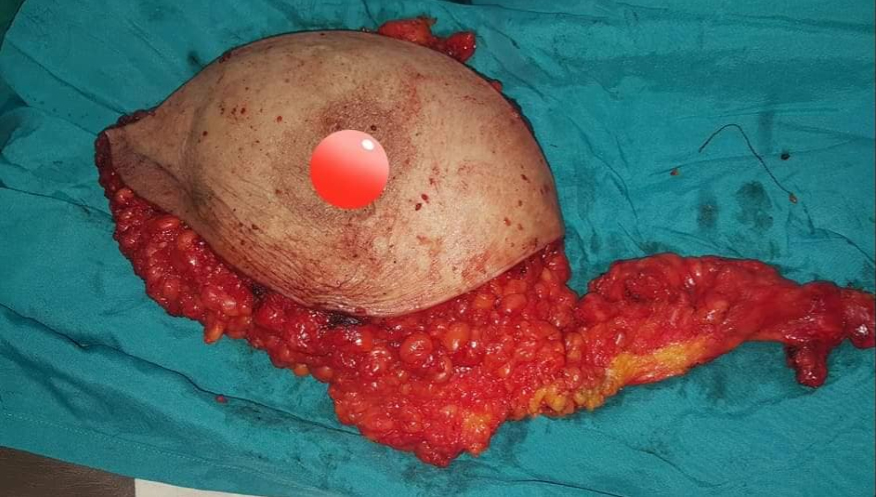
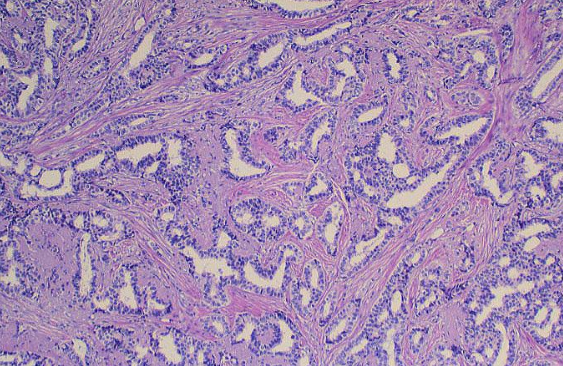
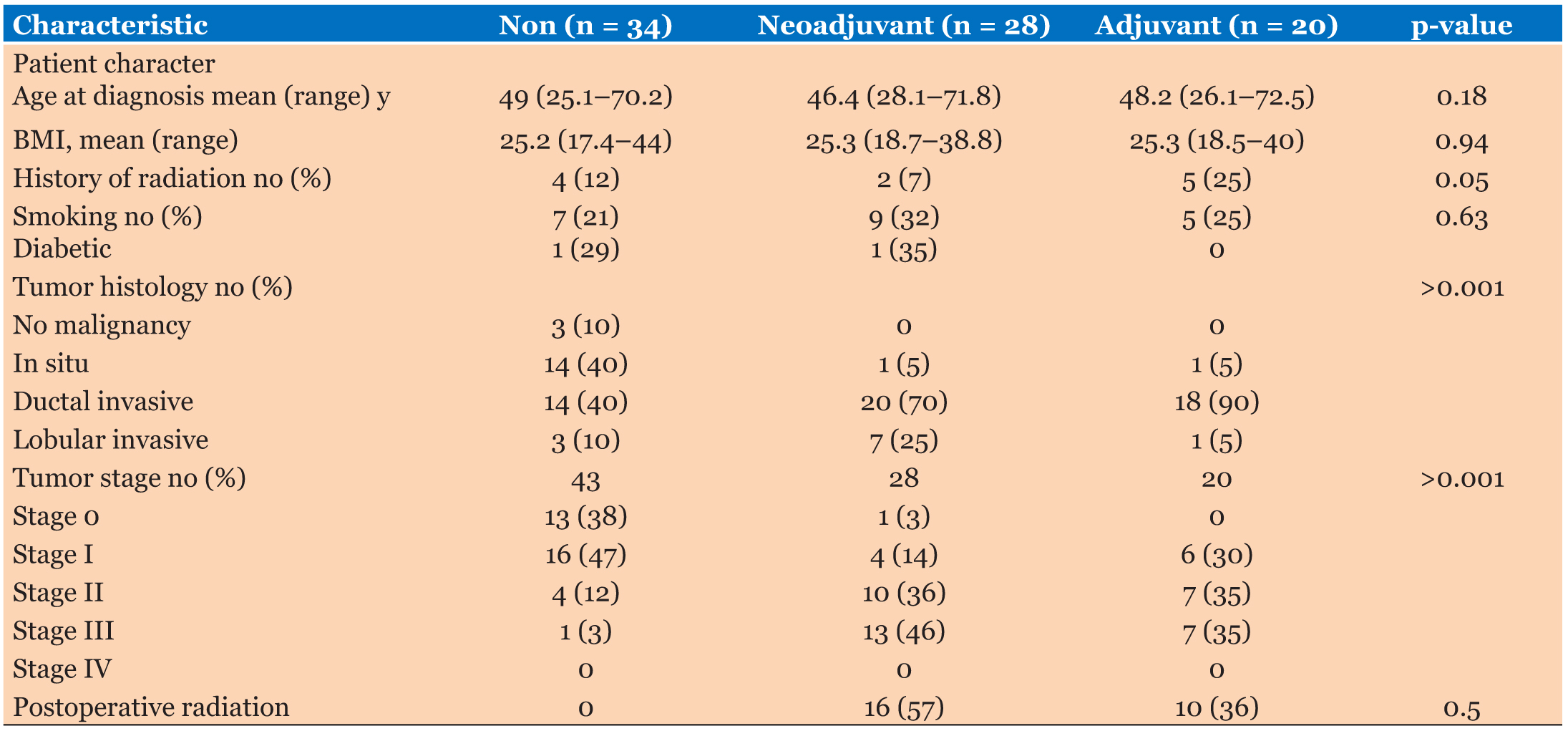
All women in our study underwent mastectomy and immediate reconstruction 6–7 weeks interval at the Zagazig University Surgical Department between January 2017 and December 2018, we had 82 female patients with average age of 25–72 years and the patients were divided into three groups, group 1: 28 patients received neoadjuvant chemotherapy, group 2: 20 patients received adjuvant chemotherapy, and group 3: 34 patients didn’t receive any chemotherapy (as shown in Table 1, 29 patients were stages 0 and 1 and out of the remaining 5 patients 3 of them were suffering of severe hepatic affection, 1 was suffering of renal failure, and 1 patient refused) (see Figure 1, Figure 2, Figure 3, Figure 4).
Patient data, present history, and treatment details collected. Surgical outcomes and complication recorded, including wound complications, skin flap (partial or full necrosis) with partial or complete loss, infections or tissue expander/implant problem, nipple necrosis, and cancer outcomes. Infectious complications included both those requiring oral antibiotics or systemic treatment in the outpatient setting and requiring hospital readmission for any cause. Reasons for unplanned return to the surgical room or reoperation included wound hematoma, wound irrigation, wound debridement for infections or necrosis, tissue expander or implant removal if associated with severe infection, incisional hernias repair or rectus diastasis after transverse rectus abdominis muscle flap reconstruction. Number of complications was included in the analysis for each of the relevant complication. All postoperative complications that occurred collected and followed up and included in the analysis. Neoadjuvant chemotherapy was defined as chemotherapy given before to the mastectomy with immediate reconstruction, while adjuvant chemotherapy was the chemotherapy given after mastectomy. All patients who received postoperative chemotherapy placed in the adjuvant group irrespective of the timing of any postoperative complication with chemotherapy initiation.
All statistical analyses were performed using the Stata version 11.0 software package. For all statistical analyses, significance was determined at the p ≤ 0.05 levels; all comparisons were two-tailed.
Exclusion criteria
Stage 4 patients with distant metastasis—bad general condition patients—patients refusing reconstruction.
RESULTS
During the study period, 82 patients underwent mastectomy and immediate breast reconstruction. Twentyeight patients received neoadjuvant chemotherapy and 20 received postoperative chemotherapy, while the rest (34 patients) did not receive any systemic therapy. Patient and tumor characteristics are described in Table 1.
Patients ranged in age from 25 to 72 years (mean, 48.2 years) at the time of mastectomy; this did not differ significantly between the groups (p = 0.18). Overall, almost 27% of patients reported a history of tobacco use, although no patient reported smoking at the time of surgery. There was no difference or significant p value in smoking groups (p = 0.63). The average body mass index was also near similar between the three groups (p = 0.94). We had only two patients who were suffering of diabetes mellitus, 1 in the neoadjuvant chemotherapy group and 1 who did not receive chemotherapy group. Thirteen percent of patients had a history of radiation therapy prior to mastectomy, with a higher percentage 25% in the adjuvant chemotherapy group, there were greater numbers of nodal positive and locally advanced tumors among the adjuvant and neoadjuvant chemotherapy groups compared with the patients who received any chemotherapy. Although the number of patients had less stage in neoadjuvant chemotherapy group than in the adjuvant chemotherapy group, this difference was with no significant value (p = 0.14). All patients routinely received prophylactic intravenous antibiotics prior to skin incision on table, typically 1 g of cefazolin or ampicillin–sulbactam but if the patients reported a penicillin allergy clindamycin or vancomycin was given. The patients in both the neoadjuvant and adjuvant chemotherapy received a standard chemotherapeutic regimen consisting of doxorubicin hydrochloride/cyclophosphamide and paclitaxel followed, including most of patients in the neoadjuvant chemotherapy group and nearly above 50% in the adjuvant chemotherapy group. Subsequent trastuzumab therapy 20% of patients in the neoadjuvant chemotherapy group and 17% of patients of adjuvant chemotherapy group additionally received. Adjuvant chemotherapy was initiated 5–6 weeks after mastectomy and immediate reconstruction performed to allow adequate time for wound healing (Table 2).
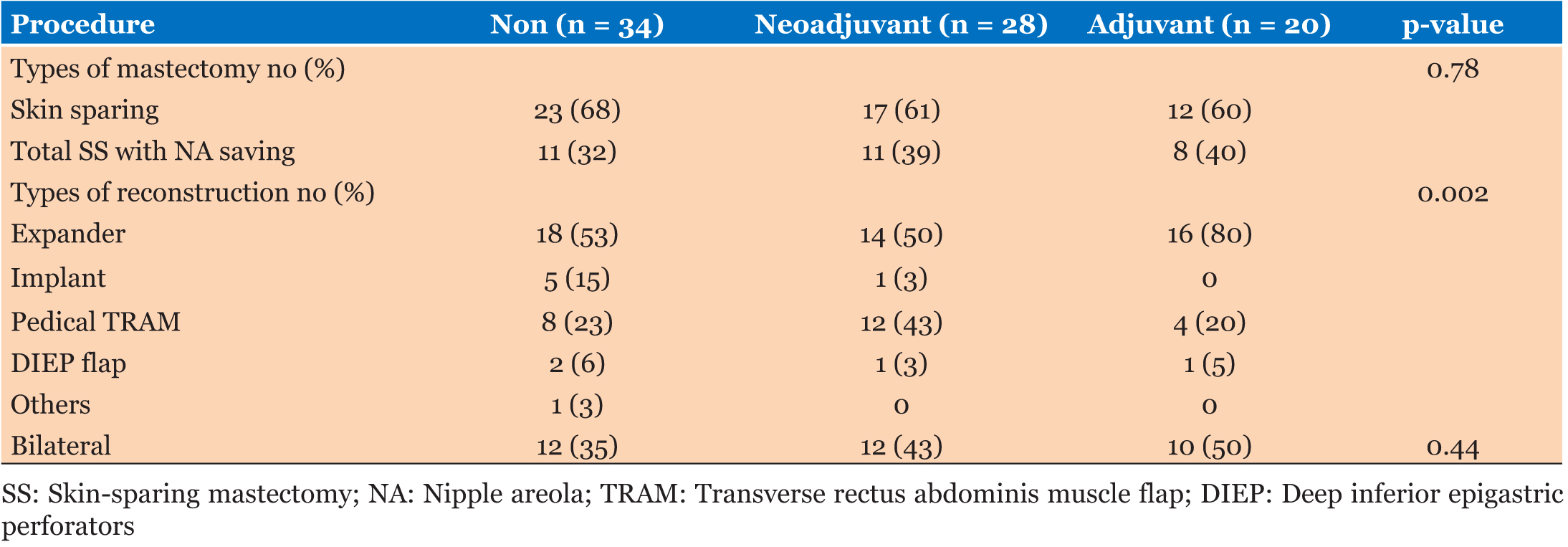
Surgical techniques included total skin-sparing mastectomy with nipple-areolar preservation, skinsparing mastectomy, and simple mastectomy. Sixty-nine percent of patients had immediate reconstruction with tissue expander placement and subsequent initial implant placement, but the rest had autologous reconstruction. Type of mastectomy had no significant value between groups (p = 0.78). However, there was significantly greater use of transverse rectus abdominis muscle reconstruction among patients with neoadjuvant chemotherapy (43%) group, but between adjuvant 20% and no chemotherapy groups 23%.
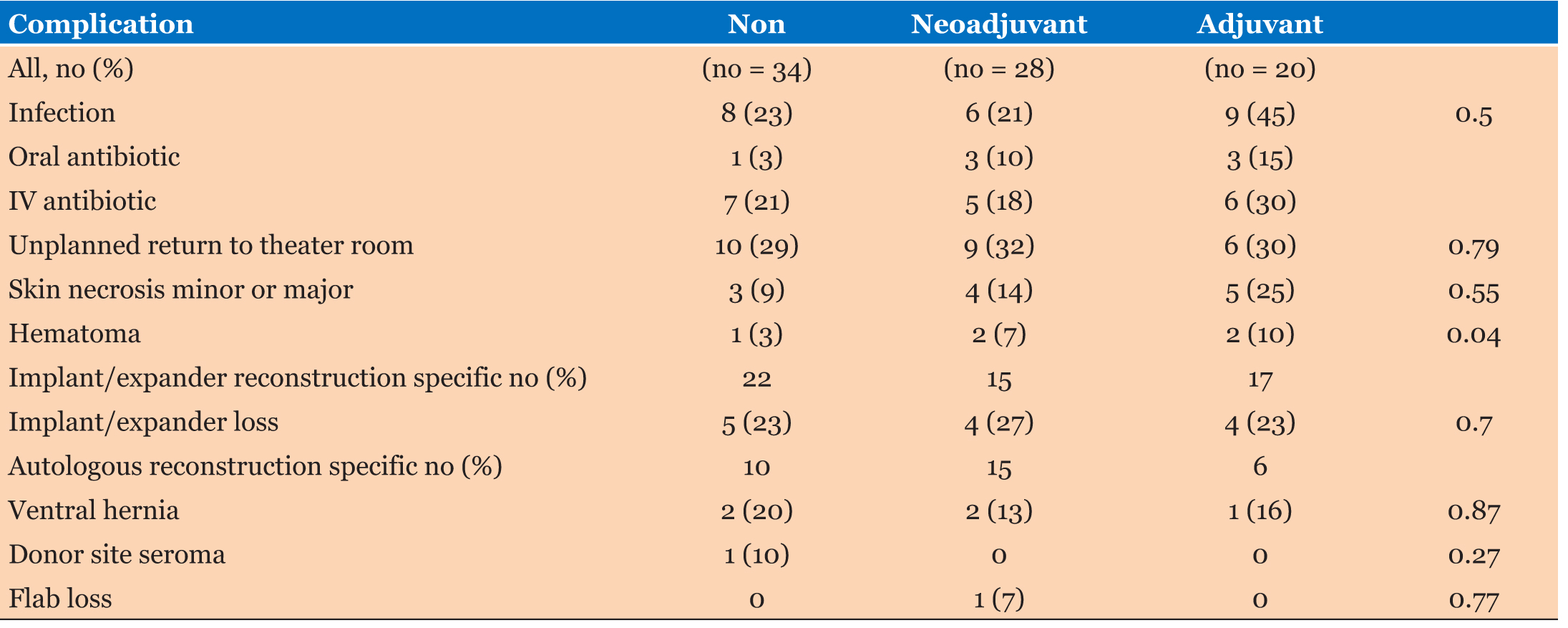
The most common postoperative complications included in Table 3. Thirty percent of patients had an unplanned return to the operating room. The most frequent indication for reintervention was tissue expander/implant removal or unplanned implant exchange with loss of expander or implant (22% of patients who underwent expander/implant reconstruction). The rate of implant loss was insignificantly between groups (p = 0.70). Fifty-seven percent of patients received neoadjuvant chemotherapy had postoperative radiation therapy, but 36% of patients treated with adjuvant chemotherapy with insignificant p value (p = 0.05). Despite this difference, the neoadjuvant chemotherapy group did not have a significantly greater implant loss rate 27%. Other indications for unplanned surgical intervention included ventral hernia repair in patients who had undergone prior transverse rectus abdominis muscle flap reconstruction (2 patients in the neoadjuvant chemotherapy, 1 in the adjuvant chemotherapy, and 2 in the group who received no chemotherapy). At a mean postoperative follow-up of 20 months (ranged from 10 to 40 months), 1 patient had locoregional recurrence and 2 patients had developed distant metastases in adjuvant group.
DISCUSSION
Many study results after mastectomy focus on the complication after mastectomy and immediate reconstruction on the role of radiation therapy, associated with a number of postoperative patients, particularly in who have undergone expander/implant reconstruction [7],[8]. Much of the discussion regarding minimizing procedure complications after immediate reconstruction has been driven by the effect of radiation therapy and has led to techniques such as delayedimmediate reconstruction [9] or approaches using a combination of autologous and prosthetic reconstruction procedure [10]. However, study the role of chemotherapy on post-reconstructive outcomes after immediate breast reconstruction has been limited and little discussed in the literature.
With the widespread use of modern neoadjuvant and adjuvant chemotherapy in patients with breast cancer disease, particularly the risk of neutropenia is significant. The development of infections in patients who are receiving adjuvant chemotherapy is high especially in patients of recently prosthetic implant procedure as part of their immediate breast reconstruction. But infectious complications is less in neoadjuvant chemotherapy group (21%) than others groups; in fact, patients in the adjuvant treatment group had the highest rate of infectious complications (45%). Moreover, we found the patients who underwent adjuvant chemotherapy as part of cancer treatment developed the postoperative complications, before initiation of chemotherapy the postoperative complication was not related to chemotherapy but related to other patient cause or surgical factors. Nevertheless, the high infection rate among patients for whom the intent was to treat with adjuvant chemotherapy is clinically important and of concern, as systemic chemotherapy was likely delayed by surgical complications in 4 patients (20%) in this group.
Multiple experimental studies done in animals showing decreased wound tensile strength after impact chemotherapy (adjuvant or neoadjuvant), particularly when adjuvant chemotherapy is given in the early postoperative period [11],[12]. However, these findings had duplicated rate in clinical trials, with several other studies showing no increased risk of wound-related complications in patients who had received neoadjuvant or adjuvant systemic therapy as compared with patients who had not received chemotherapy, supporting the findings of the current study [13],[14]. Previous studies analyzing the impact of chemotherapy on wound complications in patients with mastectomy and immediate reconstruction have no increased incidence in surgical wound complications among adjuvant chemotherapy [15],[16]. Similar results have been obtained among neoadjuvant chemotherapy patients [17],[18]. In our series the post-reconstructive wound complications including infection, skin necrosis, and seroma, ventral hernia, and hematoma were more with adjuvant chemotherapy than neoadjuvant and nonchemotherapy group, although infectious complications did not uniformly include both infections requiring oral antibiotics and intravenous antibiotics. It is more in adjuvant group rather than other group.
We found no risk of wound-related complications or increased infections in patients with neoadjuvant chemotherapy. Importantly, we do not use systemic bevacizumab, which significantly impairs normal wound healing.
McCarthy et al. [19] found no increased incidence of complications in patients with neoadjuvant or adjuvant chemotherapy compared with patients not received chemotherapy. Mitchem et al. [20] examined a series of 30 patients undergone skin-sparing mastectomy and immediate breast reconstruction with tissue expander or permanent implant placement received both neoadjuvant and adjuvant chemotherapy. They reported an overall 38% failure rate after expander/implant reconstruction because of infection, expander loss, or skin flap necrosis. In comparison to our study the percent is 23% in adjuvant and 27% in neoadjuvant but 18% in nonchemotherapy patients. Woerdeman et al. [21], [22] found a 14–20% explanation rate in their series of patients with skin-sparing mastectomy and immediate expander or permanent implant reconstruction, which is comparable to 23% rate of expander/implant loss in our study.
Given the impact of chemotherapy on wound healing demonstrated in animal models, the effect of neoadjuvant on wound after immediate reconstruction explained increased wound site complication. Despite these concerns, our results did not reveal any significant difference. Although the number of patients in each of the study groups is relatively small, the data support considering the use of neoadjuvant chemotherapy in patients who require systemic therapy as part of their breast cancer treatment. In fact, the use of neoadjuvant chemotherapy in this setting may prevent delay to systemic chemotherapy in a notable proportion of patients who develop postoperative complications. Although systemic chemotherapy has been thought to increase wound site complications, the results of our study explain the risk of noninfectious postoperative complications is not increased after mastectomy and immediate breast reconstruction between patients who received and did not receive chemotherapy.
CONCLUSION
We conclude that the patients who are planning to undergo mastectomy and immediate reconstruction, the neoadjuvant chemotherapy is a safe option that does not appear to increase the risk of postoperative wound complications. These results suggest a possible benefit from neoadjuvant chemotherapy in those patients who require chemotherapy, even in patients who will undergo mastectomy, and they support the benefit of the use of immediate reconstruction in this patient population.
REFERENCES
1.
McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol 2009;16(10):2682–90. [CrossRef]
[Pubmed]

2.
Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: Effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol 2009;27(25):4082–8. [CrossRef]
[Pubmed]

3.
Gomez SL, Lichtensztajn D, Kurian AW, et al. Increasing mastectomy rates for early-stage breast cancer? Population-based trends from California. J Clin Oncol 2010;28(10):e155–7. [CrossRef]
[Pubmed]

4.
Ueda S, Tamaki Y, Yano K, et al. Cosmetic outcome and patient satisfaction after skin-sparing mastectomy for breast cancer with immediate reconstruction of the breast. Surgery 2008;143(3):414–25. [CrossRef]
[Pubmed]

5.
Vandeweyer E, Hertens D, Nogaret JM, Deraemaecker R. Immediate breast reconstruction with saline-filled implants: No interference with the oncologic outcome? Plast Reconstr Surg 2001;107(6):1409–12. [CrossRef]
[Pubmed]

6.
Patani N, Devalia H, Anderson A, Mokbel K. Oncological safety and patient satisfaction with skin-sparing mastectomy and immediate breast reconstruction. Surg Oncol 2008;17(2):97–105. [CrossRef]
[Pubmed]

7.
Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: A critical review of the literature. Plast Reconstr Surg 2009;124(2):395–408. [CrossRef]
[Pubmed]

8.
Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: Recent trends and therapeutic implications. Plast Reconstr Surg 2000;105(3):930–42. [CrossRef]
[Pubmed]

9.
Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg 2004;113(6):1617–28. [CrossRef]
[Pubmed]

10.
Spear SL, Boehmler JH, Taylor NS, Prada C. The role of the latissimus dorsi flap in reconstruction of the irradiated breast. Plast Reconstr Surg 2007;119(1):1–9. [CrossRef]
[Pubmed]

11.
Devereux DF, Thibault L, Boretos J, Brennan MF. The quantitative and qualitative impairment of wound healing by adriamycin. Cancer 1979;43(3):932–8. [CrossRef]
[Pubmed]

12.
Lawrence WT, Talbot TL, Norton JA. Preoperative or postoperative doxorubicin hydrochloride (adriamycin): Which is better for wound healing? Surgery 1986;100(1):9–13.
[Pubmed]

13.
Meric F, Milas M, Hunt KK, et al. Impact of neoadjuvant chemotherapy on postoperative morbidity in soft tissue sarcomas. J Clin Oncol 2000;18(19):3378–83. [CrossRef]
[Pubmed]

14.
Kolb BA, Buller RE, Connor JP, DiSaia PJ, Berman ML. Effects of early postoperative chemotherapy on wound healing. Obstet Gynecol 1992;79(6):988–92.
[Pubmed]

15.
Furey PC, Macgillivray DC, Castiglione CL, Allen L. Wound complications in patients receiving adjuvant chemotherapy after mastectomy and immediate breast reconstruction for breast cancer. J Surg Oncol 1994;55(3)194–7.
[Pubmed]

16.
Mortenson MM, Schneider PD, Khatri VP, et al. Immediate breast reconstruction after mastectomy increases wound complications: However, initiation of adjuvant chemotherapy is not delayed. Arch Surg 2004;139(9):988–91. [CrossRef]
[Pubmed]

17.
Caffo O, Cazzolli D, Scalet A, et al. Concurrent adjuvant chemotherapy and immediate breast reconstruction with skin expanders after mastectomy for breast cancer. Breast Cancer Res Treat 2000;60(3):267–75.
[Pubmed]

18.
Liu Y, Mori H, Hata Y. Does neoadjuvant chemotherapy for breast cancer increase complications during immediate breast reconstruction? J Med Dent Sci 2009;56(1):55–60.
[Pubmed]

19.
McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: An outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg 2008;121(6)1886–92. [CrossRef]
[Pubmed]

20.
Mitchem J, Herrmann D, Margenthaler JA, Aft RL. Impact of neoadjuvant chemotherapy on rate of tissue expander/implant loss and progression to successful breast reconstruction following mastectomy. Am J Surg 2008;196(4):519–22. [CrossRef]
[Pubmed]

21.
Woerdeman LA, Hage JJ, Smeulders MJ, Rutgers EJ, van der Horst CM. Skin-sparing mastectomy and immediate breast reconstruction by use of implants: An assessment of risk factors for complications and cancer control in 120 patients. Plast Reconstr Surg 2006;118(2)321–30. [CrossRef]
[Pubmed]

22.
Woerdeman LA, Hage JJ, Hofland MM, Rutgers EJ. A prospective assessment of surgical risk factors in 400 cases of skin-sparing mastectomy and immediate breast reconstruction with implants to establish selection criteria. Plast Reconstr Surg 2007;119(2):455–63. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Hassan A Saad - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Ahmed M El Teliti - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Ahmed Salah Arafa - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Amr Abdelbari - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2019 Hassan A Saad et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.